Article contents
Middlebackite, a new Cu oxalate mineral from Iron Monarch, South Australia: Description and crystal structure
Published online by Cambridge University Press: 02 July 2018
Abstract
Middlebackite is a new supergene mineral formed in the upper levels of the Iron Monarch quarry, South Australia. It occurs as aggregates of blue, prismatic crystals up to 0.3 mm across comprising individual crystals up to 0.05 mm in length associated with atacamite and mottramite. Crystals are translucent with a vitreous lustre and have a pale blue streak. Middlebackite is brittle with one perfect cleavage and uneven fracture. Mohs hardness is ~2. The calculated density is 3.64 g cm–3. Crystals are biaxial (+) with α = 1.663(4), β = 1.748(4) and γ = 1.861(4) (measured in white light). The calculated 2V is 86.7°. Pleochroism is X = colourless, Y = very pale blue and Z = dark sky blue; Z > Y > X. The empirical formula unit, based on six oxygen atoms per formula unit is Cu2.00(C2O4)Cl0.02(OH)1.98. Middlebackite is monoclinic, space group P21/c with a = 7.2597(15), b = 5.7145(11), c = 5.6624(11) Å, β = 104.20(3)°, V = 227.73(8) Å3 and Z = 2. The five strongest lines in the powder X-ray diffraction pattern are [d(Å), (I), (hkl)]: 7.070 (16) (100), 3.739 (100) (11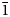 $\bar{1}$), 2.860 (18) (020), 2.481 (12) (12
$\bar{1}$), 2.860 (18) (020), 2.481 (12) (12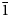 $\bar{1}$) and 2.350 (9) (300). The crystal structure was refined from synchrotron single-crystal X-ray diffraction data to R1 = 0.0341 for 596 observed reflections with F0 > 4σ(F0). The structure is based on sheets of edge- and corner-sharing octahedra parallel to the bc plane. Sheets link in the a direction via oxalate anions.
$\bar{1}$) and 2.350 (9) (300). The crystal structure was refined from synchrotron single-crystal X-ray diffraction data to R1 = 0.0341 for 596 observed reflections with F0 > 4σ(F0). The structure is based on sheets of edge- and corner-sharing octahedra parallel to the bc plane. Sheets link in the a direction via oxalate anions.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: František Laufek
References
- 2
- Cited by


