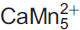 (V2O7)3·12H2O, a new vanadate mineral from the Packrat mine, near Gateway, Mesa County, Colorado, USA
(V2O7)3·12H2O, a new vanadate mineral from the Packrat mine, near Gateway, Mesa County, Colorado, USAPublished online by Cambridge University Press: 02 January 2018
Mesaite (IMA2015-069), ideally  (V2O7)3·12H2O, is a new mineral from the Packrat mine, Gateway district, Mesa County, Colorado, USA. Crystals of mesaite occur as orangish red blades up to 0.1 mm long and ∼10 μm thick. The streak is light pinkish orange and the lustre is vitreous, transparent. Mesaite has a brittle tenacity, {010} perfect cleavage; fracture is irregular, and no parting was observed. The mineral has a Mohs hardness ≈ 2. The measured density of mesaite is 2.74(1) g cm–3. Mesaite is biaxial (–), α = 1.760(calc), β = 1.780(5), γ = 1.795(5) in white light; the measured 2V value = 81(2)°. Dispersion is strong, r < v, and pleochroism is present in shades of brownish orange. Mesaite is monoclinic, P2/n, with a = 9.146(2), b = 10.424(3), c = 15.532(4) Å, β = 102.653(7)° and V = 1444.7(6) Å3. The strongest four diffraction lines in the powder diffraction pattern are [(dobs in Å, (Iobs), (hkl)]: 10.47 (100) (010), 2.881 (25) (132, 3̄12, 033, 310), 3.568 (24) (1̄14, 1̄23, 2̄13), 3.067 (17) (1̄24, 1̄32, 2̄23). The composition of mesaite was determined by electron microprobe, and yielded an empirical formula of Mn5.32Ca0.56Zn0.31V5.96As0.04O33H23.61 on the basis of 33 O atoms per formula unit (apfu).
(V2O7)3·12H2O, is a new mineral from the Packrat mine, Gateway district, Mesa County, Colorado, USA. Crystals of mesaite occur as orangish red blades up to 0.1 mm long and ∼10 μm thick. The streak is light pinkish orange and the lustre is vitreous, transparent. Mesaite has a brittle tenacity, {010} perfect cleavage; fracture is irregular, and no parting was observed. The mineral has a Mohs hardness ≈ 2. The measured density of mesaite is 2.74(1) g cm–3. Mesaite is biaxial (–), α = 1.760(calc), β = 1.780(5), γ = 1.795(5) in white light; the measured 2V value = 81(2)°. Dispersion is strong, r < v, and pleochroism is present in shades of brownish orange. Mesaite is monoclinic, P2/n, with a = 9.146(2), b = 10.424(3), c = 15.532(4) Å, β = 102.653(7)° and V = 1444.7(6) Å3. The strongest four diffraction lines in the powder diffraction pattern are [(dobs in Å, (Iobs), (hkl)]: 10.47 (100) (010), 2.881 (25) (132, 3̄12, 033, 310), 3.568 (24) (1̄14, 1̄23, 2̄13), 3.067 (17) (1̄24, 1̄32, 2̄23). The composition of mesaite was determined by electron microprobe, and yielded an empirical formula of Mn5.32Ca0.56Zn0.31V5.96As0.04O33H23.61 on the basis of 33 O atoms per formula unit (apfu).
The atomic arrangement of mesaite was solved and refined to R1 = 0.0600. The structure is formed of zigzag octahedral chains of edge-sharing Mn2+O6 octahedra. Oxygen atoms of the octahedra are shared with V2O7 groups, which link with adjacent octahedral chains to form {010} heteropolyhedral layers. The interlayer region contains Ca atoms and H2O groups. Each Ca bonds to two O6 atoms in the heteropolyhedral layer and to two fully occupied and six partially occupied O (H2O) sites in the interlayer, resulting in an effective Ca coordination of approximately seven. Similar zigzag chains of edge-sharing MnO6 octahedra decorated with V2O7 groups are also found in the mineral fianelite. Mesaite has beenapproved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2015-069). The name mesaite is conferred for Mesa County, Colorado, USA.