Article contents
Manganoarrojadite-(KNa), KNa5MnFe13Al(PO4)11(PO3OH)(OH)2, a new arrojadite-group mineral from the Palermo No. 1 mine, New Hampshire, USA
Published online by Cambridge University Press: 11 November 2020
Abstract
A new arrojadite-group mineral manganoarrojadite-(KNa), ideally KNa5MnFe13Al(PO4)11(PO3OH)(OH)2, was found in a phosphate-bearing granite pegmatite at the Palermo No. 1 mine, New Hampshire, USA. It forms anhedral grains up to 1 × 1.5 cm in size combined in aggregates with vivianite, goyazite, quartz and calcite. The mineral is olive green with a pale green streak and vitreous to greasy lustre. The cleavage is good in one direction. The Mohs hardness is 4½. Dcalc is 3.53 g/cm3. Manganoarrojadite-(KNa) is optically biaxial (–), α = 1.658(2), β = 1.666(2), γ = 1.670(2), 2Vmeas. = 67(1)° and 2Vcalc. = 70° (589 nm). The infrared spectrum is reported. The composition (wt.%) is Na2O 6.97, K2O 1.78, CaO 0.31, MgO 2.17, MnO 12.30, FeO 31.17, Al2O3 2.43, P2O5 40.48, F 0.30, H2O 1.32, O = F2 –0.13, total 99.10. The empirical formula calculated on the basis of 12 P and (O+OH+F) = 50 apfu is Na4.73K0.80Ca0.12Mg1.13Mn2+3.65Fe2+9.13Al1.00P12.00O46.59OH3.08F0.33. The ideal structural formula is A1KA2NaB1NaB2NaNa1,2Na2Na3□ CMnMFe13Al(PO4)11(PO3OH)W(OH)2. The mineral is monoclinic, Cc, a = 16.5345(3), b = 10.0406(2), c = 24.6261(5) Å, β = 105.891(2)°, V = 3932.09(14) Å3 and Z = 4. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I)(hkl)] are: 5.902(24)(202), 5.025(24)(020), 3.208(47)(206,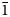 $\;\bar{1}$32), 3.048(100)(
$\;\bar{1}$32), 3.048(100)(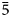 $\bar{5}$14,
$\bar{5}$14,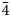 $\bar{4}$24), 2.758(24)(
$\bar{4}$24), 2.758(24)(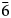 $\bar{6}$02) and 2.704(70)(226). The crystal structure, refined from single-crystal X-ray diffraction data (R1 = 0.025), is of the arrojadite structure type. Manganoarrojadite-(KNa) is the first arrojadite-group mineral with Mn dominant on the site usually occupied by Ca and without Ca as the dominant cation at any cation site.
$\bar{6}$02) and 2.704(70)(226). The crystal structure, refined from single-crystal X-ray diffraction data (R1 = 0.025), is of the arrojadite structure type. Manganoarrojadite-(KNa) is the first arrojadite-group mineral with Mn dominant on the site usually occupied by Ca and without Ca as the dominant cation at any cation site.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2020. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: G. Diego Gatta
References
- 2
- Cited by


