Published online by Cambridge University Press: 05 July 2018
The new mineral magnesiokoritnigite (IMA 2013-049), ideally Mg(AsO3OH)·H2O, was found at the Torrecillas mine, Salar Grande, Iquique Province, Chile, where it occurs as a secondary alteration phase in association with anhydrite, chudobaite, halite, lavendulan, quartz and scorodite. Crystals of magnesiokoritnigite are colourless to pale-pink, thin to thick laths up to 2 mm long. Laths are elongated on [001], flattened on {010} and exhibit the forms {010}, {110}, {1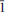 0}, {101}, {031} and {0
0}, {101}, {031} and {0 1}. The crystals also occur in dense deep-pink intergrowths. Crystals are transparent with a vitreous lustre. The mineral has a white streak, Mohs hardness of ∼3, brittle tenacity, conchoidal fracture and one perfect cleavage on {101}. The measured and calculated densities are 2.95(3) and 2.935 g cm– 3, respectively. Optically, magnesiokoritnigite is biaxial (+) with α = 1.579(1), β = 1.586(1) and γ = 1.620(1) (measured in white light). The measured 2V is 50(2)° and the calculated 2V is 50°. Dispersion is r < v, medium. The optical orientation is Y ≈ b; Z ^ c = 36° in obtuse β (note pseudomonoclinic symmetry). The mineral is non-pleochroic. The empirical formula, determined from electron-microprobe analyses, is (Mg0.94Cu0.03Mn0.02Ca0.01)Σ 1.00As0.96O5H3.19. Magnesiokoritnigite is triclinic, P
1}. The crystals also occur in dense deep-pink intergrowths. Crystals are transparent with a vitreous lustre. The mineral has a white streak, Mohs hardness of ∼3, brittle tenacity, conchoidal fracture and one perfect cleavage on {101}. The measured and calculated densities are 2.95(3) and 2.935 g cm– 3, respectively. Optically, magnesiokoritnigite is biaxial (+) with α = 1.579(1), β = 1.586(1) and γ = 1.620(1) (measured in white light). The measured 2V is 50(2)° and the calculated 2V is 50°. Dispersion is r < v, medium. The optical orientation is Y ≈ b; Z ^ c = 36° in obtuse β (note pseudomonoclinic symmetry). The mineral is non-pleochroic. The empirical formula, determined from electron-microprobe analyses, is (Mg0.94Cu0.03Mn0.02Ca0.01)Σ 1.00As0.96O5H3.19. Magnesiokoritnigite is triclinic, P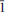 , with a = 7.8702(7), b = 15.8081(6), c = 6.6389(14) Å, α = 90.814(6), β = 96.193(6), γ = 90.094(7)°, V = 821.06(19) Å3 and Z = 8. The eight strongest X-ray powder diffraction lines are [dobs Å (I)(hkl)]: 7.96(100)(020), 4.80(54)(101), 3.791(85)(
, with a = 7.8702(7), b = 15.8081(6), c = 6.6389(14) Å, α = 90.814(6), β = 96.193(6), γ = 90.094(7)°, V = 821.06(19) Å3 and Z = 8. The eight strongest X-ray powder diffraction lines are [dobs Å (I)(hkl)]: 7.96(100)(020), 4.80(54)(101), 3.791(85)(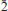 10,210,
10,210,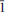
 1,
1,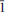 31), 3.242(56)(0
31), 3.242(56)(0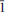 2,
2,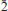
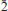 1,012), 3.157(92)(2
1,012), 3.157(92)(2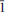 1,
1,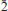 30,230), 3.021(61)(1
30,230), 3.021(61)(1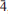 1,141,2
1,141,2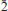 1,221), 2.798(41)(0
1,221), 2.798(41)(0 2,032) and 1.908(43)(multiple). The structure, refined to R1 = 5.74% for 2360 Fo > 4σF reflections, shows magnesiokoritnigite to be isostructural with koritnigite and cobaltkoritnigite.
2,032) and 1.908(43)(multiple). The structure, refined to R1 = 5.74% for 2360 Fo > 4σF reflections, shows magnesiokoritnigite to be isostructural with koritnigite and cobaltkoritnigite.