Published online by Cambridge University Press: 25 November 2020
Jeankempite, Ca5(AsO4)2(AsO3OH)2(H2O)7, is a new mineral species (IMA2018-090) discovered amongst coatings of arsenate minerals on oxidised copper arsenides from the Mohawk No. 2 mine, Mohawk, Keweenaw County, Michigan, USA. The new mineral occurs as lamellar bundles of colourless to white plates up to 1 mm wide and is visually indistinguishable from guérinite, with which it forms intergrowths. Jeankempite is transparent to translucent with a waxy lustre and white streak, is non-fluorescent under longwave and shortwave ultraviolet illumination, has a Mohs hardness of ~1.5 and brittle tenacity with uneven fracture. Crystals are flattened on {01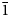 $\bar{1}$} and exhibit perfect cleavage on {01
$\bar{1}$} and exhibit perfect cleavage on {01 $\bar{1}$}. Optically, jeankempite is biaxial (+), α = 1.601(2), β = 1.607(2), γ = 1.619(2) (white light); 2Vmeas. = 72(2)° and 2Vcalc. = 71.0°. The empirical formula is (Ca4.97Na0.013Mg0.017)(As3.99S0.01)4O23H16, based on 23 O and 16 H atoms per formula unit. Thermogravimetric analysis indicates that jeankempite undergoes four weight losses totalling 16.82%, close to the expected loss of 16.30%, corresponding to eight H2O. Jeankempite is triclinic, P
$\bar{1}$}. Optically, jeankempite is biaxial (+), α = 1.601(2), β = 1.607(2), γ = 1.619(2) (white light); 2Vmeas. = 72(2)° and 2Vcalc. = 71.0°. The empirical formula is (Ca4.97Na0.013Mg0.017)(As3.99S0.01)4O23H16, based on 23 O and 16 H atoms per formula unit. Thermogravimetric analysis indicates that jeankempite undergoes four weight losses totalling 16.82%, close to the expected loss of 16.30%, corresponding to eight H2O. Jeankempite is triclinic, P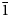 $\bar{1}$, a = 6.710(6), b = 14.901(14), c = 15.940(15) Å, α = 73.583(12)°, β = 81.984(12)°, γ = 82.754(12)°, V = 1507(2) Å3 and Z = 3. The final structure was refined to R1 = 0.0591 for 2781 reflections with Iobs > 3σI. The crystal structure of jeankempite is built from a network of edge- and vertex-sharing CaO6, CaO7 and AsO4 polyhedra, and we hypothesise that the new mineral has formed due to a topotactic reaction brought on by dehydration of preexisting guérinite.
$\bar{1}$, a = 6.710(6), b = 14.901(14), c = 15.940(15) Å, α = 73.583(12)°, β = 81.984(12)°, γ = 82.754(12)°, V = 1507(2) Å3 and Z = 3. The final structure was refined to R1 = 0.0591 for 2781 reflections with Iobs > 3σI. The crystal structure of jeankempite is built from a network of edge- and vertex-sharing CaO6, CaO7 and AsO4 polyhedra, and we hypothesise that the new mineral has formed due to a topotactic reaction brought on by dehydration of preexisting guérinite.
Associate Editor: Michael Rumsey