Article contents
From structure topology to chemical composition. XIV. Titanium silicates: refinement of the crystal structure and revision of the chemical formula of mosandrite, (Ca3REE)[(H2O)2Ca0.5□0.5]Ti(Si2O7)2(OH)2(H2O)2, a Group-I mineral from the Saga mine, Morje, Porsgrunn, Norway
Published online by Cambridge University Press: 05 July 2018
Abstract
The crystal structure of mosandrite, ideally (Ca3REE)[(H2O)2Ca0.5☐0.5]Ti(Si2O7)2(OH)2(H2O)2, from the Saga mine, Morje, Porsgrunn, Norway, has been refined as two components related by the TWIN matrix (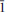 0 0, 0
0 0, 0 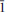 0, 1 0 1): a 7.4222(3), b 5.6178(2), c 18.7232(7) Å, β 101.4226(6)°, V = 765.23(9) Å3, space group P21/c, Dcalc. = 3.361 g.cm–3, R1 = 3.69% using 1347 observed (Fo > 4σF) reflections. The empirical formula of mosandrite (EMPA) was calculated on the basis of 4 Si a.p.f.u., with H2O determined from structure refinement: [(Ca2.89Ba0.01)Σ2.90(Ce0.39La0.18Nd0.14Sm0.02Gd0.03Y0.16Th0.03)Σ1.01Zr0.09]Σ4 [(H2O)2.00Ca0.32Na0.17Al0.10Mn0.04Fe2+0.02☐0.35]Σ3(Ti0.87Nb0.09Zr0.04)Σ1(Si2O7)2[(OH)1.54F0.46]Σ2[(H2O)1.50F0.50]Σ2, Z = 2. The crystal structure of mosandrite is a framework of TS (titanium silicate) blocks; each TS block consists of HOH sheets (H-heteropolyhedral, O-octahedral). In the TS block, there are five fully occupied cation sites, two [4]-coordinated Si sites with <Si–O> 1.623 Å , [7]-coordinated MH and AP sites occupied by Ca and REE in the ratio ∼3:1, and one [6]-coordinated Ti-dominant MO(1) site. There are two H2O-dominant H2O-alkali-cation sites. The partly occupied MO(2) site has composition [(H2O)0.5☐0.33Na0.17], ideally [(H2O)0.5☐0.5] p.f.u. The MO(3) site has ideal composition [(H2O)1.5Ca0.5] p.f.u. In the O sheet, the XOM and XOA anion sites have compositions [(OH)1.54F0.46] (XOM) and [(H2O)1.50F0.50] (XOA), ideally (OH)2 and (H2O)2 p.f.u. The MH and AP polyhedra and Si2O7 groups constitute the H sheet that is completely ordered. In the O sheet, MO(1) octahedra are long-range ordered whereas H2O and OH groups and alkali cations Na and Ca are long-range disordered. Two SRO (short-range ordered) arrangements have been proposed for the O sheet: (1) Na [MO(2)], Ca2 [MO(3)] and F4[XOM and XOA anion sites]; (2) 2 H2O [MO(2)] and MO(3)] and (OH)2 and (H2O)2 [XOM and XOA]. Linkage of H and O sheets occurs mainly via common vertices of MH polyhedra and Si2O7 groups and MO(1) octahedra. Two adjacent TS blocks are related by the glide plane cy. Mosandrite is an H2O- and OH-bearing Na- and Ca-depleted analogue of rinkite, ideally (Ca3REE)Na(NaCa) Ti(Si2O7)2(OF)F2. Mosandrite and rinkite are related by the following substitution at the MO(2,3) and XO(M,A) sites in the O sheet: M[(H2O)2 + ☐0.5] + X[(OH)–2 + (H2O)2] ↔ M[Na+2 + Ca2+0.5] + X[(OF)3– + (F2)2–].
0, 1 0 1): a 7.4222(3), b 5.6178(2), c 18.7232(7) Å, β 101.4226(6)°, V = 765.23(9) Å3, space group P21/c, Dcalc. = 3.361 g.cm–3, R1 = 3.69% using 1347 observed (Fo > 4σF) reflections. The empirical formula of mosandrite (EMPA) was calculated on the basis of 4 Si a.p.f.u., with H2O determined from structure refinement: [(Ca2.89Ba0.01)Σ2.90(Ce0.39La0.18Nd0.14Sm0.02Gd0.03Y0.16Th0.03)Σ1.01Zr0.09]Σ4 [(H2O)2.00Ca0.32Na0.17Al0.10Mn0.04Fe2+0.02☐0.35]Σ3(Ti0.87Nb0.09Zr0.04)Σ1(Si2O7)2[(OH)1.54F0.46]Σ2[(H2O)1.50F0.50]Σ2, Z = 2. The crystal structure of mosandrite is a framework of TS (titanium silicate) blocks; each TS block consists of HOH sheets (H-heteropolyhedral, O-octahedral). In the TS block, there are five fully occupied cation sites, two [4]-coordinated Si sites with <Si–O> 1.623 Å , [7]-coordinated MH and AP sites occupied by Ca and REE in the ratio ∼3:1, and one [6]-coordinated Ti-dominant MO(1) site. There are two H2O-dominant H2O-alkali-cation sites. The partly occupied MO(2) site has composition [(H2O)0.5☐0.33Na0.17], ideally [(H2O)0.5☐0.5] p.f.u. The MO(3) site has ideal composition [(H2O)1.5Ca0.5] p.f.u. In the O sheet, the XOM and XOA anion sites have compositions [(OH)1.54F0.46] (XOM) and [(H2O)1.50F0.50] (XOA), ideally (OH)2 and (H2O)2 p.f.u. The MH and AP polyhedra and Si2O7 groups constitute the H sheet that is completely ordered. In the O sheet, MO(1) octahedra are long-range ordered whereas H2O and OH groups and alkali cations Na and Ca are long-range disordered. Two SRO (short-range ordered) arrangements have been proposed for the O sheet: (1) Na [MO(2)], Ca2 [MO(3)] and F4[XOM and XOA anion sites]; (2) 2 H2O [MO(2)] and MO(3)] and (OH)2 and (H2O)2 [XOM and XOA]. Linkage of H and O sheets occurs mainly via common vertices of MH polyhedra and Si2O7 groups and MO(1) octahedra. Two adjacent TS blocks are related by the glide plane cy. Mosandrite is an H2O- and OH-bearing Na- and Ca-depleted analogue of rinkite, ideally (Ca3REE)Na(NaCa) Ti(Si2O7)2(OF)F2. Mosandrite and rinkite are related by the following substitution at the MO(2,3) and XO(M,A) sites in the O sheet: M[(H2O)2 + ☐0.5] + X[(OH)–2 + (H2O)2] ↔ M[Na+2 + Ca2+0.5] + X[(OF)3– + (F2)2–].
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2013
References
- 9
- Cited by


