Article contents
Description and crystal structure of bobkingite,  a new mineral from New Cliffe Hill Quarry, Stanton-under-Bardon, Leicestershire, UK
a new mineral from New Cliffe Hill Quarry, Stanton-under-Bardon, Leicestershire, UK
Published online by Cambridge University Press: 05 July 2018
Abstract
Bobkingite, ideally 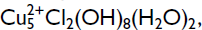 is a new mineral from the New Cliffe Hill Quarry, Stantonunder-Bardon, Leicestershire, England. It occurs as very thin (⩽5 µm) transparent plates up to 0.2 mm across, perched on a compact fibrous crust of malachite and crystalline azurite attached to massive cuprite. Crystals are tabular on {001} with dominant {001} and minor {100} and {110}. Bobkingite is a soft pale blue colour with a pale-blue streak, vitreous lustre and no observable fluorescence under ultraviolet light. It has perfect {001} and fair {100} cleavages, no observable parting, conchoidal fracture, and is brittle. Its Mohs' hardness is 3 and the calculated density is 3.254 g/cm3. Bobkingite is biaxial negative with α = 1.724(2), β = 1.745(2), γ = 1.750(2), 2Vγmeas = 33(6)°, 2Vcalc = 52°, pleochroism distinct, X = very pale blue, Z = pale greenish blue, X^a = 22° (in β obtuse), Y = c, Z = b. Bobkingite is monoclinic, space group C2/m, unit-cell parameters (refined from powder data): a = 10.301(8), b = 6.758(3), c = 8.835(7)Å, β = 111.53(6)°, V = 572.1(7)Å3, Z = 2. The seven strongest lines in the X-ray powder-diffraction pattern are [d (Å), I, (hkl)]: 8.199, 100, (001); 5.502, 100, (110); 5.029, 40, (
is a new mineral from the New Cliffe Hill Quarry, Stantonunder-Bardon, Leicestershire, England. It occurs as very thin (⩽5 µm) transparent plates up to 0.2 mm across, perched on a compact fibrous crust of malachite and crystalline azurite attached to massive cuprite. Crystals are tabular on {001} with dominant {001} and minor {100} and {110}. Bobkingite is a soft pale blue colour with a pale-blue streak, vitreous lustre and no observable fluorescence under ultraviolet light. It has perfect {001} and fair {100} cleavages, no observable parting, conchoidal fracture, and is brittle. Its Mohs' hardness is 3 and the calculated density is 3.254 g/cm3. Bobkingite is biaxial negative with α = 1.724(2), β = 1.745(2), γ = 1.750(2), 2Vγmeas = 33(6)°, 2Vcalc = 52°, pleochroism distinct, X = very pale blue, Z = pale greenish blue, X^a = 22° (in β obtuse), Y = c, Z = b. Bobkingite is monoclinic, space group C2/m, unit-cell parameters (refined from powder data): a = 10.301(8), b = 6.758(3), c = 8.835(7)Å, β = 111.53(6)°, V = 572.1(7)Å3, Z = 2. The seven strongest lines in the X-ray powder-diffraction pattern are [d (Å), I, (hkl)]: 8.199, 100, (001); 5.502, 100, (110); 5.029, 40, ( 01); 2.883, 80, (310); 2.693, 40, (
01); 2.883, 80, (310); 2.693, 40, (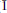 13); 2.263, 40, (113), (
13); 2.263, 40, (113), (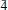 03); 2.188, 50, (
03); 2.188, 50, ( 23). Chemical analysis by electron microprobe and crystal-structure solution and refinement gave CuO 70.46, Cl 12.71, H2O 19.19, O≡Cl –2.87, sum 99.49 wt.%, where the amount of H2O was determined by crystal-structure analysis. The resulting empirical formula on the basis of 12 anions (including 8 (OH) and 2H2O) is Cu4.99Cl2.02O10H12. The crystal structure was solved by direct methods and refined to an R index of 2.6% for 638 observed reflections measured with X-rays on a single crystal. Three distinct (Cuϕ6) (ϕ = unspecified anion) octahedra share edges to form a framework that is related to the structures of paratacamite and the Cu2(OH)3Cl polymorphs, atacamite and clinoatacamite. The mineral is named for Robert King, formerly of the Department of Geology, Leicester University, prominent mineral collector and founding member of the Russell Society. The mineral and its name have been approved by the Commission on New Minerals and Mineral Names of the International Mineralogical Association.
23). Chemical analysis by electron microprobe and crystal-structure solution and refinement gave CuO 70.46, Cl 12.71, H2O 19.19, O≡Cl –2.87, sum 99.49 wt.%, where the amount of H2O was determined by crystal-structure analysis. The resulting empirical formula on the basis of 12 anions (including 8 (OH) and 2H2O) is Cu4.99Cl2.02O10H12. The crystal structure was solved by direct methods and refined to an R index of 2.6% for 638 observed reflections measured with X-rays on a single crystal. Three distinct (Cuϕ6) (ϕ = unspecified anion) octahedra share edges to form a framework that is related to the structures of paratacamite and the Cu2(OH)3Cl polymorphs, atacamite and clinoatacamite. The mineral is named for Robert King, formerly of the Department of Geology, Leicester University, prominent mineral collector and founding member of the Russell Society. The mineral and its name have been approved by the Commission on New Minerals and Mineral Names of the International Mineralogical Association.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2002
References
- 11
- Cited by


