Article contents
Description and crystal structure of a new mineral – plimerite, ZnFe3+4(PO4)3(OH)5 – the Zn-analogue of rockbridgeite and frondelite, from Broken Hill, New South Wales, Australia
Published online by Cambridge University Press: 05 July 2018
Abstract
Plimerite, ideally Zn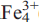 (PO4)3(OH)5, is a new mineral from the Block 14 Opencut, Broken Hill, New SouthWales. It occurs as pale-green to dark-olive-green, almost black, acicular to prismatic and bladed crystals up to 0.5 mm long and as hemispherical aggregates of radiating acicular crystals up to 3 mm across. Crystals are elongated along [001] and the principal form observed is {100} with minor {010} and {001}. The mineral is associated with hinsdalite-plumbogummite, pyromorphite, libethenite, brochantite, malachite, tsumebite and strengite. Plimerite is translucent with a pale-greyish-green streak and a vitreous lustre. It shows an excellent cleavage parallel to {100} and {010} and distinct cleavage parallel to {001}. It is brittle, has an uneven fracture, a Mohs’ hardness of 3.5–4, D(meas.) = 3.67(5) g/cm3 and D(calc.) = 3.62 g/cm3 (for the empirical formula). Optically, it is biaxial negative with α = 1.756(5), β = 1.764(4), γ = 1.767(4) and 2V(calc.) of –63º; pleochroism is X pale-greenish-brown, Y pale-brown, Z pale-bluish-green; absorption Z > X > Y; optical orientation XYZ = cab. Plimerite is orthorhombic, space group Bbmm, unit-cell parameters: a = 13.865(3) Å, b = 16.798(3) Å, c = 5.151(10) Å, V = 1187.0(4) Å3 (single-crystal data) and Z = 4. Strongest lines in the X-ray powder diffraction pattern are [d (A˚ ), I, hkl]: 4.638, (50), (111); 3.388, (50), (041); 3.369, (55), (131); 3.168, (100), (132); 2.753, (60), (115); 2.575, (90), (200); 2.414, (75), (220); 2.400, (50), (221); 1.957, (40), (225). Electron microprobe analysis yielded (wt.%): PbO 0.36, CaO 0.17, ZnO 20.17, MnO 0.02, Fe2O3 29.82, FeO 2.98, Al2O3 4.48, P2O5 32.37, As2O5 0.09, H2O (calc) 6.84, total 97.30 (Fe3+/Fe2+ ratio determined by Mössbauer spectroscopy). The empirical formula calculated on the basis of 17 oxygens is Ca0.02Pb0.01Zn1.68
(PO4)3(OH)5, is a new mineral from the Block 14 Opencut, Broken Hill, New SouthWales. It occurs as pale-green to dark-olive-green, almost black, acicular to prismatic and bladed crystals up to 0.5 mm long and as hemispherical aggregates of radiating acicular crystals up to 3 mm across. Crystals are elongated along [001] and the principal form observed is {100} with minor {010} and {001}. The mineral is associated with hinsdalite-plumbogummite, pyromorphite, libethenite, brochantite, malachite, tsumebite and strengite. Plimerite is translucent with a pale-greyish-green streak and a vitreous lustre. It shows an excellent cleavage parallel to {100} and {010} and distinct cleavage parallel to {001}. It is brittle, has an uneven fracture, a Mohs’ hardness of 3.5–4, D(meas.) = 3.67(5) g/cm3 and D(calc.) = 3.62 g/cm3 (for the empirical formula). Optically, it is biaxial negative with α = 1.756(5), β = 1.764(4), γ = 1.767(4) and 2V(calc.) of –63º; pleochroism is X pale-greenish-brown, Y pale-brown, Z pale-bluish-green; absorption Z > X > Y; optical orientation XYZ = cab. Plimerite is orthorhombic, space group Bbmm, unit-cell parameters: a = 13.865(3) Å, b = 16.798(3) Å, c = 5.151(10) Å, V = 1187.0(4) Å3 (single-crystal data) and Z = 4. Strongest lines in the X-ray powder diffraction pattern are [d (A˚ ), I, hkl]: 4.638, (50), (111); 3.388, (50), (041); 3.369, (55), (131); 3.168, (100), (132); 2.753, (60), (115); 2.575, (90), (200); 2.414, (75), (220); 2.400, (50), (221); 1.957, (40), (225). Electron microprobe analysis yielded (wt.%): PbO 0.36, CaO 0.17, ZnO 20.17, MnO 0.02, Fe2O3 29.82, FeO 2.98, Al2O3 4.48, P2O5 32.37, As2O5 0.09, H2O (calc) 6.84, total 97.30 (Fe3+/Fe2+ ratio determined by Mössbauer spectroscopy). The empirical formula calculated on the basis of 17 oxygens is Ca0.02Pb0.01Zn1.68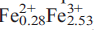 Al0.60P3.09As0.01O17.00H5.15. The crystal structure was solved by direct methods and refined to an R1 index of 6.41% for 1332 observed reflections from single-crystal X-ray diffraction data (Mo-Kα radiation, CCD area detector). The structure of plimerite is isotypic with that of rockbridgeite and is based on face-sharing trimers of (Mϕ6) octahedra which link by sharing edges to form chains, that extend in the b-direction. Chains link to clusters comprising pairs of corner-sharing (Mϕ6) octahedra that link to PO4 tetrahedra forming sheets parallel to (001). The sheets link via octahedra and tetrahedra corners into a heteropolyhedral framework structure. The mineral name honours Professor Ian Plimer for his contributions to the study of the geology of ore deposits.
Al0.60P3.09As0.01O17.00H5.15. The crystal structure was solved by direct methods and refined to an R1 index of 6.41% for 1332 observed reflections from single-crystal X-ray diffraction data (Mo-Kα radiation, CCD area detector). The structure of plimerite is isotypic with that of rockbridgeite and is based on face-sharing trimers of (Mϕ6) octahedra which link by sharing edges to form chains, that extend in the b-direction. Chains link to clusters comprising pairs of corner-sharing (Mϕ6) octahedra that link to PO4 tetrahedra forming sheets parallel to (001). The sheets link via octahedra and tetrahedra corners into a heteropolyhedral framework structure. The mineral name honours Professor Ian Plimer for his contributions to the study of the geology of ore deposits.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2009
References
- 9
- Cited by


