Article contents
The crystal structure of voggite, a new hydrated Na–Zr hydroxide–phosphate–carbonate mineral*
Published online by Cambridge University Press: 05 July 2018
Abstract
The crystal structure of the new mineral voggite, Na2Zr(PO4)(CO3)(OH).2H2O , from the Francon quarry, Montreal, Quebec, Canada, has been solved in order to determine the correct chemical formula, as conventional electron microprobe methods were found unreliable. The unit cell is monoclinic, I2/m, with a = 12.261(2), b = 6.561(1), c = 11.757(2)Å, β = 116.19(2)°. The structure consists of layers of edge-sharing Zr-O pentagonal bipyramids, separated by layers of Na-(O,H2O) octahedra. The carbonate ion acts as a bidentate ligand in the Zr-O polyhedron, the third oxygen atom being bonded to the Na atom. The phosphate group is bonded to three different Zr atoms and to a Na atom. The Zr-O bond lengths vary from 2.067 to 2.283 (mean 2.140Å), while Na-O are between 2.304 and 2.773, (σ = 0.006Å, mean 2.480Å). The carbonate and phosphate bonds are normal. It is inferred from the structure that the columns of octahedrally coordinated Na atoms can easily be broken apart when subjected to the heat generated by the electron microprobe beam, with the subsequent expulsion of water. This gives rise to ‘mobile’ Na atoms, which make quantitative electron microprobe analysis extremely difficult. The structure allows the ‘liberated’ Na atoms to move freely within planes parallel to 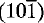 .
.
Keywords
- Type
- Crystal Structures
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1990
References
- 1
- Cited by


