Article contents
Crystal structure of Na4[Cu4O2(SO4)4]·MeCl (Me: Na, Cu, ☐) – the synthetic Na-analogue of piypite (caratiite)
Published online by Cambridge University Press: 05 July 2018
Abstract
The crystal structure of Na4[Cu4O2(SO4)4] MeCl (Me: Na,Cu,☐) has been solved by direct methods and refined to R1 = 0.028 using 2466 independent reflections. The compound is tetragonal with space group P4/n, a = 18.451(1)Å, c = 4.9520(2)Å, Z = 4. The two main building units running parallel to the c-axis comprise: (1) slabs containing SO4 and OCu4 tetrahedra; and (2) chains of corner sharing Me(Cl,O)6 octahedra. The displacement ellipsoids of the atoms occupying the Me sites and of the Cl ions indicate a short-range static disorder. Copper in the slabs exhibits a (4+1) coordination. Both elements form a tunnel structure in which additional Na atoms are incorporated for charge compensation. The structure is closely related to the mineral piypite (K4Cu4O2(SO4)4 MeCl (Me: Na,Cu,☐)): 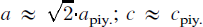 . Furthermore, the structure has a pronounced pseudo-translational symmetry: apart from some of the oxygen sites, all of the nineteen crystallographically distinct positions are related by a pseudo F centring.
. Furthermore, the structure has a pronounced pseudo-translational symmetry: apart from some of the oxygen sites, all of the nineteen crystallographically distinct positions are related by a pseudo F centring.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2000
References
- 6
- Cited by


