No CrossRef data available.
Article contents
Chenite, Pb4Cu(SO4)2(OH)6, a new mineral, from Leadhills, Scotland
Published online by Cambridge University Press: 05 July 2018
Abstract
Chenite, a new lead-copper secondary mineral, has been found on specimens from the Leadhills area, Scotland. It is associated with caledonite, linarite, leadhillite, susannite, and other species, on oxidized galena with chalcopyrite. Electron microprobe analysis yielded PbO 74.5, CuO 7.8, SO3 13.3, H2O 4.4 (by difference), sum = 100 wt. %. The empirical formula (based on 14 oxygens) is Pb3.98Cu1.17S1.98O14H5.82; the ideal formula is Pb4Cu(SO4)2(OH)6, which requires PbO 75.2, CuO 6.7, SO3 13.5, H2O 4.6, sum = 100 wt. %.
Infra-red spectroscopy showed the presence of only 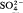 and OH− ions, with no H2O.
and OH− ions, with no H2O.
Chenite is triclinic, P1 or P ̄, with a = 5.791(1), b = 7.940(1), c = 7.976(1) Å, α = 112.02(1), β = 97.73(1), γ = 100.45(1)°, V = 326.0 Å3, Z = 1. The strongest lines in the X-ray powder diffraction pattern (d, I/Io, hkl) are: 5.55, 7, 100; 4.32, 6, 1
̄, with a = 5.791(1), b = 7.940(1), c = 7.976(1) Å, α = 112.02(1), β = 97.73(1), γ = 100.45(1)°, V = 326.0 Å3, Z = 1. The strongest lines in the X-ray powder diffraction pattern (d, I/Io, hkl) are: 5.55, 7, 100; 4.32, 6, 1 1; 3.60, 10 002; 3.41, 9, 1
1; 3.60, 10 002; 3.41, 9, 1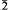 0; 3.30, 5, 02
0; 3.30, 5, 02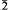 ; 3.00, 5, 111; 2.80, 7, 12
; 3.00, 5, 111; 2.80, 7, 12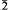 ; 2.07, 6, 211/21
; 2.07, 6, 211/21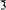 /1
/1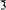 3; 1.778, 5, 3
3; 1.778, 5, 3
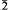 /2
/2 3.
3.
Chenite forms minute, singly terminated, transparent to translucent sky-blue crystals from 0.1 to over 1 mm long, elongated approximately [032]. Twenty different forms (pinacoids) have been identified on the four crystals studied. A good cleavage on {100}, and traces of a second on {001}, can be observed. Optically, chenite is biaxial negative, 2 V(measured) = 67±1°, 2 V(calc.) = 68° (Na). The refractive indices are α 1.871±0.005, β 1.909±0.005, γ 1.927±0.005 (Na). Dispersion is strong, r≫v. The mineral is weakly pleochroic. H (Mohs) ∼ 2½. D = 5.98, and calculated Dx = 6.044 g cm−3.
Keywords
- Type
- Mineralogy
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1986


