Published online by Cambridge University Press: 05 July 2018
Bendadaite, ideally Fe2+Fe23+ (AsO4)2(OH)2·4H2O, is a new member of the arthurite group. It was found as a weathering product of arsenopyrite on a single hand specimen from the phosphate pegmatite Bendada, central Portugal (type locality). Co-type locality is the granite pegmatite of Lavra do Almerindo (Almerindo mine), Linópolis, Divino das Laranjeiras county, Minas Gerais, Brazil. Further localities are the Veta Negra mine, Copiapó province, Chile; Oumlil-East, Bou Azzer district, Morocco; and Pira Inferida yard, Fenugu Sibiri mine, Gonnosfanadiga, Medio Campidano Province, Sardinia, Italy.
Type bendadaite occurs as blackish green to dark brownish tufts (<0.1 mm long) and flattened radiating aggregates, in intimate association with an intermediate member of the scorodite–mansfieldite series. It is monoclinic, space group P21/c, with a = 10.239(3) Å, b = 9.713(2) Å, c = 5.552(2) Å, β = 94.11(2)°, V = 550.7(2) Å3, Z = 2. Electron-microprobe analysis yielded (wt.%): CaO 0.04, MnO 0.03, CuO 0.06, ZnO 0.04, Fe2O3 (total) 43.92, Al2O3 1.15, SnO2 0.10, As2O5 43.27, P2O5 1.86, SO3 0.03. The empirical formula is (Fe2+0.52Fe3+0.32☐0.16)Σ1.00(Fe3+1.89Al0.11)Σ2.00(As1.87P0.13)Σ2.00O8(OH)2.00·4H2O based on 2(As,P) and assuming ideal 8O, 2(OH), 4H2O and complete occupancy of the ferric iron site by Fe3+ and Al. Optically, bendadaite is biaxial, positive, 2Vest. = 85±4°, 2Vcalc. = 88°, with α 1.734(3), β 1.759(3), γ 1.787(4). Pleochroism is medium strong: X pale reddish brown, Y yellowish brown, Z dark yellowish brown; absorption Z > Y > X, optical dispersion weak, r > v. Optical axis plane is parallel to (010), with X approximately parallel to a and Z nearly parallel to c. Bendadaite has vitreous to sub-adamantine luster, is translucent and non-fluorescent. It is brittle, shows irregular fracture and a good cleavage parallel to {010}. Dmeas. 3.15±0.10 g/cm3, Dcalc. 3.193 g/cm3 (for the empirical formula). The five strongest powder diffraction lines [d in Å (I)(hkl)] are 10.22 (10)(100), 7.036 (8)(110), 4.250 (5)(111), 2.865 (4)(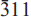 ), 4.833 (3)(020,011). The d spacings are very similar to those of its Zn analogue, ojuelaite. The crystal structure of bendadaite was solved and refined using a crystal from the co-type locality with the composition (Fe2+0.95☐0.05)Σ1.00(Fe3+1.80Al0.20)Σ2.00(As1.48P0.52)Σ2.00O8(OH)2·4H2O (R = 1.6%), and confirms an arthurite-type atomic arrangement.
), 4.833 (3)(020,011). The d spacings are very similar to those of its Zn analogue, ojuelaite. The crystal structure of bendadaite was solved and refined using a crystal from the co-type locality with the composition (Fe2+0.95☐0.05)Σ1.00(Fe3+1.80Al0.20)Σ2.00(As1.48P0.52)Σ2.00O8(OH)2·4H2O (R = 1.6%), and confirms an arthurite-type atomic arrangement.
To send this article to your Kindle, first ensure no-reply@cambridge.org is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about sending to your Kindle. Find out more about saving to your Kindle.
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox.
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive.