Published online by Cambridge University Press: 07 June 2023
The new mineral bakakinite, ideally Ca2V2O7, was found in the high-temperature (not lower than 500°C) exhalations of the Arsenatnaya fumarole at the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption, Tolbachik volcano, Kamchatka, Russia. It is associated with anhydrite, svabite, pliniusite, schäferite, berzeliite, diopside, hematite, powellite, baryte, fluorapatite, calciojohillerite, ludwigite, magnesioferrite, anorthite, titanite and esseneite. Bakakinite forms flattened crystals up to 30 × 5 μm, typically distorted. The mineral is transparent, colourless or pale yellow, with strong vitreous lustre. Electron microprobe analysis gave (wt.%): CaO 37.04, SrO 0.26, SiO2 0.16, P2O5 1.48, V2O5 49.47, As2O5 10.85, SO3 0.35, total 99.61. The empirical formula calculated on the basis of 7 O apfu is (Ca1.99Sr0.01)Σ2.00(V1.64As0.28P0.06Si0.01S0.01)Σ2.00O7. The Dcalc is 3.463 g cm–3. Bakakinite is triclinic, P$\bar{1}$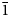 , unit-cell parameters are: a = 6.64(2), b = 6.92(2), c = 7.01(2) Å, α = 86.59(7), β = 63.77(7), γ = 83.47(6)°, V = 287.0(5) Å3 and Z = 2. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I)(hkl)] are: 4.647(27)(111, 0$\bar{1}$
, unit-cell parameters are: a = 6.64(2), b = 6.92(2), c = 7.01(2) Å, α = 86.59(7), β = 63.77(7), γ = 83.47(6)°, V = 287.0(5) Å3 and Z = 2. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I)(hkl)] are: 4.647(27)(111, 0$\bar{1}$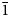 1), 3.138(76)(002), 3.103(100)(120, 121), 3.027(20)(021), 2.960(81)(200), 2.158(19)(031, 302), 1.791(16)(320), 1.682(16)(114) and 1.584(17)(1$\bar{3}$
1), 3.138(76)(002), 3.103(100)(120, 121), 3.027(20)(021), 2.960(81)(200), 2.158(19)(031, 302), 1.791(16)(320), 1.682(16)(114) and 1.584(17)(1$\bar{3}$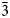 3, 403). Bakakinite is a natural analogue of synthetic Ca2V2O7. The mineral is named in honour of the outstanding Russian crystallographer and crystal chemist Vladimir Vasilievich Bakakin (born 1933).
3, 403). Bakakinite is a natural analogue of synthetic Ca2V2O7. The mineral is named in honour of the outstanding Russian crystallographer and crystal chemist Vladimir Vasilievich Bakakin (born 1933).
Associate Editor: Anthony R. Kampf