Published online by Cambridge University Press: 05 July 2018
The new mineral species grandaite, ideally Sr2Al(AsO4)2(OH), has been discovered on the dump of Valletta mine, Maira Valley, Cuneo province, Piedmont, Italy. Its origin is related to the reaction between the ore minerals and hydrothermal solutions. It occurs in thin masses of bright orange to salmon to brown coloured crystals, or infrequently as fan-like aggregates of small (<1 mm) crystals, with reddish-brown streak and waxy to vitreous lustre. Grandaite is associated with aegirine, baryte, braunite, hematite, tilasite, quartz, unidentified Mn oxides and Mn silicates under study.
Grandaite is biaxial (+) with refractive indices α = 1.726(1), β = 1.731(1), γ = 1.752(1). Its calculated density is 4.378 g/cm3. Grandaite is monoclinic, space group P21/m, with a = 7.5764(5), b = 5.9507(4), c = 8.8050(6) Å, β = 112.551(2)°, V = 366.62(4) Å3 and Z = 2. The eight strongest diffraction lines of the observed X-ray powder diffraction pattern are [d in Å, (I), (hkl)]: 3.194 (100)(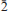 11), 2.981 (50.9)(020), 2.922 (40.2)(
11), 2.981 (50.9)(020), 2.922 (40.2)(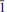 03), 2.743 (31.4)(120), 2.705 (65.2)(112), 2.087 (51.8) (
03), 2.743 (31.4)(120), 2.705 (65.2)(112), 2.087 (51.8) (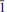 23), 1.685 (24.5)(321), 1.663 (27.7)(132). Chemical analyses by electron microprobe gave (wt.%) SrO 29.81, CaO 7.28, BaO 1.56, Al2O3 7.07, Fe2O3 2.34, Mn2O3 1.88, MgO 1.04, PbO 0.43, As2O5 44.95, V2O5 0.50, P2O5 0.09, sum 96.95; H2O 1.83 wt.% was calculated by stoichiometry from the results of the crystal-structure analysis. Raman and infrared spectroscopies confirmed the presence of (AsO4)3− and OH groups. The empirical formula calculated on the basis of 9 O a.p.f.u., in agreement with the structural results, is (Sr1.41Ca0.64Ba0.05Pb0.01)∑=2.11(Al0.68Fe0.143+Mn0.123+Mg0.13)∑=1.07 [(As0.96V0.01)∑=0.97O4]2(OH), the simplified formula is (Sr,Ca)2(Al,Fe3+)(AsO4)2(OH) and the ideal formula is Sr2Al(AsO4)2(OH).
23), 1.685 (24.5)(321), 1.663 (27.7)(132). Chemical analyses by electron microprobe gave (wt.%) SrO 29.81, CaO 7.28, BaO 1.56, Al2O3 7.07, Fe2O3 2.34, Mn2O3 1.88, MgO 1.04, PbO 0.43, As2O5 44.95, V2O5 0.50, P2O5 0.09, sum 96.95; H2O 1.83 wt.% was calculated by stoichiometry from the results of the crystal-structure analysis. Raman and infrared spectroscopies confirmed the presence of (AsO4)3− and OH groups. The empirical formula calculated on the basis of 9 O a.p.f.u., in agreement with the structural results, is (Sr1.41Ca0.64Ba0.05Pb0.01)∑=2.11(Al0.68Fe0.143+Mn0.123+Mg0.13)∑=1.07 [(As0.96V0.01)∑=0.97O4]2(OH), the simplified formula is (Sr,Ca)2(Al,Fe3+)(AsO4)2(OH) and the ideal formula is Sr2Al(AsO4)2(OH).
The crystal structure was solved by direct methods and found to be topologically identical to that of arsenbrackebuschite. The structure model was refined on the basis of 1442 observed reflections to R1 = 2.78%. In the structure of grandaite, chains of edge-sharing M3+ octahedra run along [010] and share vertices with T5+ tetrahedra, building up [M3+(T5+O4)2(OH, H2O)] units, which are connected through interstitial divalent cations. Grandaite is named after the informal appellation of the province where the type locality is located. The new mineral was approved by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification (IMA2013-059). The discovery of grandaite and of other members of the group (description still in progress) opens up the possibility of exploring the crystal chemistry of the brackebuschite supergroup.