Published online by Cambridge University Press: 05 July 2018
Alcaparrosaite, ideally K3Ti4+Fe3+(SO4)4O(H2O)2, is a new mineral from the Alcaparrosa mine, Cerritos Bayos, El Loa Province, Antofagasta, Chile (IMA2011-024). The mineral occurs on and intergrown with coquimbite, and is also associated with ferrinatrite, krausite, pertlikite, pyrite, tamarugite and voltaite. It is a relatively early phase which forms during the oxidation of pyritic masses under increasingly arid conditions. Alcaparrosaite crystallizes from hyperacidic solutions in a chemical environment that is consistent with its association with coquimbite. It occurs as pale yellow blades and tapering prisms up to 4 mm in length, flattened on {010} and elongated along [100]. The observed crystal forms are {010}, {110}, {1.13.0} and {021}. The mineral is transparent and has a white streak, vitreous lustre, Mohs hardness of about 4, brittle tenacity, conchoidal fracture and no cleavage. The measured and calculated densities are 2.80(3) and 2.807 g cm–3, respectively. It is optically biaxial (+) with α = 1.643(1), β = 1.655(1), γ = 1.680(1) (white light), 2Vmeas = 70(2)° and 2Vcalc = 70.3°. The mineral exhibits strong parallel dispersion, r < v. The optical orientation is X = b; Y^c = 27° in the obtuse angle β. No pleochroism was observed. Electron-microprobe analyses (average of 4) provided: Na2O 0.32, K2O 20.44, Fe2O3 11.58, TiO2 11.77, P2O50.55, SO347.52, H2O 5.79 (calc); total 97.97 wt.%. The empirical formula (based on 19 O) is (K2.89Na0.07)Σ2.96Ti0.984+Fe0.973+(S0.99P0.01O4)4O0.72(OH)0.28(H2O)2. The mineral is hydrophobic, insoluble in cold and hot water, very slowly soluble in acids and decomposes slowly in bases. Alcaparrosaite is monoclinic, C2/c, with the cell parameters a = 7.55943(14), b = 16.7923(3), c = 12.1783(9) Å, β = 94.076(7)°, V = 1542.01(12) Å3 and Z = 4. The eight strongest lines in the X-ray powder diffraction pattern [dobs in A ˚ (Irel) (hkl)] are 6.907 (41) (021,110); 3.628 (34) (023,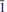 13); 3.320 (32) (
13); 3.320 (32) (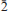 02); 3.096 (100) (202,
02); 3.096 (100) (202,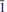 33,150); 3.000 (40) (
33,150); 3.000 (40) (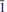 51); 2.704 (38) (
51); 2.704 (38) (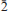 23,152); 1.9283 (30) (
23,152); 1.9283 (30) (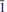 55); 1.8406 (31) (
55); 1.8406 (31) ( 53,
53,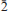 06). In the structure of alcaparrosaite (R1 = 2.57% for 1725 Fo > 4σF), Ti4+ and Fe3+, in roughly equal amounts, occupy the same octahedrally coordinated site. Octahedra are linked into dimers by corner sharing. The SO4 tetrahedra link the dimers into chains parallel to [001] and link the chains into undulating sheets parallel to {010}. The sheets link via 10- and 11-coordinated K atoms in the interlayer region. The structure shares some features with that of goldichite.
06). In the structure of alcaparrosaite (R1 = 2.57% for 1725 Fo > 4σF), Ti4+ and Fe3+, in roughly equal amounts, occupy the same octahedrally coordinated site. Octahedra are linked into dimers by corner sharing. The SO4 tetrahedra link the dimers into chains parallel to [001] and link the chains into undulating sheets parallel to {010}. The sheets link via 10- and 11-coordinated K atoms in the interlayer region. The structure shares some features with that of goldichite.