Article contents
The structure of reyerite, (Na,K)2Ca14Si22Al2O58(OH)8.6H2O
Published online by Cambridge University Press: 05 July 2018
Abstract
The crystal structure of reyerite, (Na,K)2Ca14Si22Al2O58(OH)8.6H2O, Z = 1, was refined in the space group P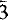 , a = 9.765, c = 19.067Å, to R = 0.064 for 1540 reflections. The structure is composed of the following structural units: (a) tetrahedral sheets S1, with composition (Si8O20)8−, characterized by six-membered rings of tetrahedra; (b) tetrahedral sheets S2, characterized by six-membered rings of tetrahedra, with six tetrahedra pointing in one direction and two pointing in the other direction—the apical oxygens of these two tetrahedra connect two inversion-related S2 sheets to build
, a = 9.765, c = 19.067Å, to R = 0.064 for 1540 reflections. The structure is composed of the following structural units: (a) tetrahedral sheets S1, with composition (Si8O20)8−, characterized by six-membered rings of tetrahedra; (b) tetrahedral sheets S2, characterized by six-membered rings of tetrahedra, with six tetrahedra pointing in one direction and two pointing in the other direction—the apical oxygens of these two tetrahedra connect two inversion-related S2 sheets to build 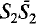 double sheets, with composition (Si14Al2O38)14− and ordered distribution of aluminum cations; (c) sheets O of edge-sharing calcium octahedra. The various structural units are connected through corner sharing according to the schematic sequence …
double sheets, with composition (Si14Al2O38)14− and ordered distribution of aluminum cations; (c) sheets O of edge-sharing calcium octahedra. The various structural units are connected through corner sharing according to the schematic sequence …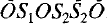 …; the corresponding composition is [Ca14Si22Al2O58(OH)8]2−. The charge balance is restored by alkali cations which are placed, together with water molecules, in the cavities of the structure at the level of the double tetrahedral sheet.
…; the corresponding composition is [Ca14Si22Al2O58(OH)8]2−. The charge balance is restored by alkali cations which are placed, together with water molecules, in the cavities of the structure at the level of the double tetrahedral sheet.
- Type
- Crystallograhy
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1988
References
A correction has been issued for this article:
- 16
- Cited by
Linked content
Please note a has been issued for this article.


