No CrossRef data available.
Published online by Cambridge University Press: 28 June 2019
Potassic-jeanlouisite, ideally K(NaCa)(Mg4Ti)Si8O22O2, is the first characterised species of oxo amphibole related to the sodium–calcium group, and derives from potassic richterite via the coupled exchange CMg–1W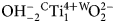 ${\rm OH}_{{\rm \ndash 2}}^{\ndash}{} ^{\rm C}{\rm Ti}_1^{{\rm 4 +}} {} ^{\rm W}\!{\rm O}_2^{2\ndash} $. The mineral and the mineral name were approved by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification, IMA2018-050. Potassic-jeanlouisite was found in a specimen of leucite which is found in the lava layers, collected in the active gravel quarry on Zirkle Mesa, Leucite Hills, Wyoming, USA. It occurs as pale yellow to colourless acicular crystals in small vugs. The empirical formula derived from electron microprobe analysis and single-crystal structure refinement is: A(K0.84Na0.16)Σ1.00B(Ca0.93Na1.02Mg0.04
${\rm OH}_{{\rm \ndash 2}}^{\ndash}{} ^{\rm C}{\rm Ti}_1^{{\rm 4 +}} {} ^{\rm W}\!{\rm O}_2^{2\ndash} $. The mineral and the mineral name were approved by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification, IMA2018-050. Potassic-jeanlouisite was found in a specimen of leucite which is found in the lava layers, collected in the active gravel quarry on Zirkle Mesa, Leucite Hills, Wyoming, USA. It occurs as pale yellow to colourless acicular crystals in small vugs. The empirical formula derived from electron microprobe analysis and single-crystal structure refinement is: A(K0.84Na0.16)Σ1.00B(Ca0.93Na1.02Mg0.04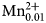 ${\rm Mn}_{{\rm 0}{\rm. 01}}^{2 +} $)Σ2.00C(Mg3.85
${\rm Mn}_{{\rm 0}{\rm. 01}}^{2 +} $)Σ2.00C(Mg3.85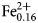 ${\rm Fe}_{{\rm 0}{\rm. 16}}^{2 +} $Ni0.01
${\rm Fe}_{{\rm 0}{\rm. 16}}^{2 +} $Ni0.01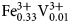 ${\rm Fe}_{{\rm 0}{\rm. 33}}^{3 +} {\rm V}_{{\rm 0}{\rm. 01}}^{3 +} $Ti0.65)Σ5.01T(Si7.76Al0.09Ti0.15)Σ8.00O22W[O1.53F0.47]Σ2.00. The holotype crystal is biaxial (–), with α = 1.674(2), β = 1.688(2), γ = 1.698(2), 2Vmeas. = 79(1)° and 2Vcalc. = 79.8°. The unit-cell parameters are a = 9.9372(10), b = 18.010(2), c = 5.2808(5) Å, β = 104.955(2)°, V = 913.1(2) Å3, Z = 2 and space group C2/m. The strongest eight reflections in the powder X-ray pattern [d values (in Å) (I) (hkl)] are: 2.703 (100) (151); 3.380 (87) (131); 2.541 (80) (
${\rm Fe}_{{\rm 0}{\rm. 33}}^{3 +} {\rm V}_{{\rm 0}{\rm. 01}}^{3 +} $Ti0.65)Σ5.01T(Si7.76Al0.09Ti0.15)Σ8.00O22W[O1.53F0.47]Σ2.00. The holotype crystal is biaxial (–), with α = 1.674(2), β = 1.688(2), γ = 1.698(2), 2Vmeas. = 79(1)° and 2Vcalc. = 79.8°. The unit-cell parameters are a = 9.9372(10), b = 18.010(2), c = 5.2808(5) Å, β = 104.955(2)°, V = 913.1(2) Å3, Z = 2 and space group C2/m. The strongest eight reflections in the powder X-ray pattern [d values (in Å) (I) (hkl)] are: 2.703 (100) (151); 3.380 (87) (131); 2.541 (80) (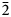 $\bar 2$02); 3.151 (70) (310); 3.284 (68) (240); 8.472 (59) (110); 2.587 (52) (061); 2.945 (50) (221,
$\bar 2$02); 3.151 (70) (310); 3.284 (68) (240); 8.472 (59) (110); 2.587 (52) (061); 2.945 (50) (221,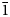 $\bar 1$51).
$\bar 1$51).
Associate Editor: Anthony R Kampf