Published online by Cambridge University Press: 05 July 2018
Meurigite is a new hydrated potassium iron phosphate related to kidwellite and with structural similarities to other late-stage fibrous ferric phosphate species. It has been found at four localities so far — the Santa Rita mine, New Mexico, U.S.A.; the Hagendorf-Sud pegmatite in Bavaria, Germany; granite pegmatite veins at Wycheproof, Victoria, Australia; and at the Gold Quarry Mine, Nevada, U.S.A. The Santa Rita mine is the designated type locality. Meurigite occurs as tabular, elongated crystals forming spherical and hemispherical clusters and drusy coatings. The colour ranges from creamy white to pale yellow and yellowish brown. At the type locality, the hemispheres may reach 2 mm across, but the maximum diameter reached in the other occurrences is usually less than 0.5 mm. A wide variety of secondary phosphate minerals accompanies meurigite at each locality, with dufrenite, cyrilovite, beraunite, rockbridgeite and leucophosphite amongst the most common. Vanadates and uranates occur with meurigite at the Gold Quarry mine.
Electron microprobe analysis and separate determination of H2O and CO2 on meurigite from the type locality gave a composition for which several empirical formulae could be calculated. The preferred formula, obtained on the basis of 35 oxygen atoms, is 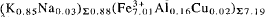
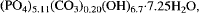 which simplies
which simplies  Qualitative analyses only were obtained for meurigite from the other localities, due to the softness and openness of the aggregates. Because of the fibrous nature of meurigite, it was not possible to determine the crystal structure, hence the exact stoichiometry remains uncertain. The lustre of meurigite varies from vitreous to waxy for the Santa Rita mine mineral, to silky for the more open sprays and internal surfaces elsewhere. The streak is very pale yellow to cream and the estimated Mohs hardness is about 3. Cleavage is perfect on {001} and fragments from the type material have a mean specific gravity of 2.96.
Qualitative analyses only were obtained for meurigite from the other localities, due to the softness and openness of the aggregates. Because of the fibrous nature of meurigite, it was not possible to determine the crystal structure, hence the exact stoichiometry remains uncertain. The lustre of meurigite varies from vitreous to waxy for the Santa Rita mine mineral, to silky for the more open sprays and internal surfaces elsewhere. The streak is very pale yellow to cream and the estimated Mohs hardness is about 3. Cleavage is perfect on {001} and fragments from the type material have a mean specific gravity of 2.96.
The strongest lines in the X-ray powder pattern for the type material are (dobs,Iobs,hkl) 3.216(100)404; 4.84(90)111; 3.116(80)205; 4.32(70)112; 9.41(60)201; 3.470(60)800. The X-ray data were indexed on the basis of a monoclinic unit cell determined from electron diffraction patterns. The cell parameters, refined by least squares methods, are a = 29.52(4), b = 5.249(6), c = 18.26(1) Å, β = 109.27(7)°, V = 2672(3) Å3, and Z = 4. The calculated density is 2.89 gcm−3. The space group is either C2, Cm or C2/m. X-ray powder data for meurigite are closely similar to those for kidwellite and phosphofibrite, but meurigite appears to be characterised by a strong 14 Å reflection. The relationship between these three minerals remains uncertain in the absence of structural data. On the available evidence, meurigite and kidwellite are not the respective K and Na-endmembers of a solid solution series. The meurigite cell parameters suggest it belongs to a structural family of fibrous ferric phosphates, such as rockbridgeite, dufrenite and beraunite, which have a discrete 5 Å fibre axis. Meurigite occurs in widely varying environments, its formation probably favoured by late-stage solutions rich in K rather than Na.