Article contents
Medvedevite, KMn2+V5+2O6Cl⋅2H2O, a new fumarolic mineral from the Tolbachik fissure eruption 2012–2013, Kamchatka Peninsula, Russia
Published online by Cambridge University Press: 13 May 2022
Abstract
Medvedevite, KMn2+V5+2O6Cl⋅2H2O, is a new mineral discovered in the Toludskoe lava field, formed during the 2012–2013 Tolbachik fissure eruption. The mineral occurs as thin acicular transparent bright red crystals up to 0.15 mm. Medvedevite is associated with thénardite, halite, aphthitalite, leonite, kieserite, eugsterite and syngenite. The empirical formula calculated on the basis of 13+ positive charge units for the anhydrous part and 2H2O is (K1.02Na0.03)Σ1.05Mn2+0.95(V5+1.92S6+0.05Si0.04)Σ2.01O6.02Cl0.96⋅2H2O. The crystal structure of medvedevite was determined using single-crystal X-ray diffraction data: monoclinic crystal system, the space group is P21/c, a = 7.1863(2), b = 10.1147(3), c = 12.7252(4) Å, β = 106.243(3)°, V = 888.04(5) Å3, Z = 4 and R1 = 0.029. The concept of ‘structural unit’ and ‘interstitial complex’ could be applied to the crystal structure of medvedevite. The structural units in medvedevite are based on the high bond-valence V5+O5 polyhedra which share edges and link into [V2O6] chains elongated along the a axis. The interstitial complexes consist of Mn2+, K+ cations and H2O groups and occupy the interstices between structural units. The mineral is optically biaxial (+), with α =1.782(2), β = 1.786(2), γ = 1.792(2), 2V(calc) = 41° (λ = 589 nm). The seven strongest lines of the powder XRD pattern are [d, Å (I, %) (hkl)]: 7.79(100)(011); 5.70(11)(110); 4.75(14)(11$\bar{2}$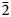 ); 3.89(29)(022); 3.25(53)(031); 2.958(79)(21$\bar{3}$
); 3.89(29)(022); 3.25(53)(031); 2.958(79)(21$\bar{3}$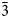 ); and 2.850(33)(220). The mineral has been named in honour of the Russian geologist and chemist Robert Alexandrovich Medvedev (1939–2005).
); and 2.850(33)(220). The mineral has been named in honour of the Russian geologist and chemist Robert Alexandrovich Medvedev (1939–2005).
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: G. Diego Gatta
References
- 1
- Cited by


