Published online by Cambridge University Press: 05 July 2018
The mineral deerite is restricted in occurrence to blueschist facies meta-ironstones. From the ideal formula of 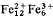 Si12O40(OH)10 there are a limited range of substitutions: Ti and V for Fe3+ and Mn on the Fe2+ site. In the present survey of deerite compositions from the majority of deerite localities the maximum substitutions have been 2Ti and 3Mn in the above formula. There is an at present unexplained anomaly in the totals of a number of the analyses indicating that there may be variations away from the ideal formula towards increased hydroxyl and decreased cation contents.
Si12O40(OH)10 there are a limited range of substitutions: Ti and V for Fe3+ and Mn on the Fe2+ site. In the present survey of deerite compositions from the majority of deerite localities the maximum substitutions have been 2Ti and 3Mn in the above formula. There is an at present unexplained anomaly in the totals of a number of the analyses indicating that there may be variations away from the ideal formula towards increased hydroxyl and decreased cation contents.
From differences between the mineral compatibilities of deerites from the higher-grade Franciscan exotic block blueschist localities and those from more conformable Alpine blueschists, in conjunction with previous work on the experimental stabilities of the low-pressure/low-temperature hydrous iron silicate minerals, it has been possible to map the form of the low-pressure deerite stability field involving reactions of greenalite or minnesotaite or grunerite with magnetite and quartz. Within the deerite stability field, further deerite-forming reactions involve the breakdown of riebeckite and the hydrous incompatibility of magnetite with quartz; both are very dependent on the activity of sodium. A low-pressure stability to 4 kb at 200 °C and 6 kb at 300 °C are estimated from these low-temperature breakdown reactions. This fits in well with the high-pressure deerite stability determinations of Langer et al. (1977).