Article contents
High-temperature diffusion of oxygen in synthetic diopside measured by nuclear reaction analysis
Published online by Cambridge University Press: 05 July 2018
Abstract
We have performed O self-diffusion experiments in synthetic diopside single crystals along the b-axis at temperatures ranging from 1473–1643 K, under controlled O partial pressure (10−11–10−2 atm). The 18O tracer diffusion was imposed by solid/gas exchange between 16O in diopside and 18O2-enriched argon-hydrogen-H2O gas mixture. Diffusion profiles of 18O were measured by Nuclear Reaction Analysis 18O (p,α) 15N. The diffusion coefficients are described by 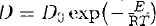 , with log D0(m2/s)=−9.2 ± 1.0 and E 310 ± 30 kJ/mol.
, with log D0(m2/s)=−9.2 ± 1.0 and E 310 ± 30 kJ/mol.
Our results are in agreement with Ryerson and McKeegan's (1994) data and Farver's (1989) data along a direction perpendicular to the c direction. Experiments performed in a wide pO2 range show that D is independent of pO2.
We observe no change in the diffusion regime up to 1643 K (i.e. 22 K prior to melting temperature). This result differs from the diffusion study of Ca in diopside by Dimanov and Ingrin (1995), where a strong enhancement of Ca mobility, attributed to an excess disorder in the Ca-sublattice, was observed above 1523 K. We conclude that O diffusion in diopside is not affected by this premelting phenomenon.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1999
References
- 8
- Cited by


