Published online by Cambridge University Press: 05 July 2018
Two new lead oxychloride minerals, hereroite [Pb32(O, ☐)21](AsO4)2((Si,As,V,Mo)O4)2Cl10 and vladkrivovichevite [Pb32O18][Pb4Mn2O]Cl14(BO3)8·2H2O occur in association with asisite, damaraite, kombatite, sahlinite, copper, quartz, barysilite, Mn silicates and a number of Mn oxyhydroxide minerals on a specimen from the Kombat mine in Namibia. The minerals formed as late-stage products of hydrothermal reworking of primary sulfide minerals.
Hereroite is monoclinic, C2/c with a = 23.14(1), b = 22.65(1), c = 12.39(1) Å, β = 102.00(5)°, V = 6351.6(41) Å3 from powder-diffraction data and a = 23.139(4), b = 22.684(4), c = 12.389(2) Å, β = 102.090(3)°, V = 6358.8(18) Å3 from single-crystal data. It is bright orange, with white streak and adamantine lustre. It is brittle with no observed parting or cleavage and has a conchoidal fracture. The calculated density is 8.15 g cm–3. The mean refractive index in air at 589 nm is 2.38. The six strongest reflections in the X-ray powder diffraction pattern [d in Å, (I), (hkl)] are as follows: 2.982(100)(5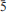 1); 2.795(47)(80
1); 2.795(47)(80 ); 1.986(24)(8
); 1.986(24)(8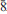
 ); 1.641(24)(11.
); 1.641(24)(11.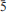 .
.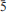 ); 3.512(23)(61
); 3.512(23)(61 ); 3.901(21)(511). Hereroite is named for the Herero people, one of the indigenous tribal groupings in the region where the Kombat mine is located.
); 3.901(21)(511). Hereroite is named for the Herero people, one of the indigenous tribal groupings in the region where the Kombat mine is located.
Vladkrivovichevite is orthorhombic, Pmmn with a = 12.87(5), b = 27.7(4), c = 11.46(3) Å, V = 4080.1(5) Å3, from powder-diffraction data and a = 12.759(1), b = 27.169(4), c = 11.515(1) Å, V = 3992.0(9) Å3, Z = 2, from single-crystal data. It is pale greenish yellow, with white streak and adamantine lustre. It is brittle with no observed parting or cleavage and has a conchoidal fracture. The calculated density is 7.40 g cm–3. The mean refractive indices in air at 589 nm are 2.30 and 2.34. The six strongest reflections in the X-ray powder diffraction pattern [d in Å, (I), (hkl)] are as follows: 2.860(100)(370); 2.733(84)(073); 3.707(49)(073); 3.068(37)(401); 2.075(32)(473); 1.601(32)(3.14.3). Vladkrivovichevite is named in honour of Prof. Dr Vladimir Gerasimovich Krivovichev (b. 24.04.1946), Head of the Department of Mineralogy, Geological Faculty, St Petersburg State University.
The crystal structures of hereroite and vladkrivovichevite consist of alternating litharge-like O – Pb double layers and chlorine sheets and both are structurally related to other layered lead oxychlorides. In hereroite, tetrahedral AsO4 and (Si, As, V, Mo)O4 groups locate in defects within the O – Pb block, which combines square 'symesite-type' and double-square 'kombatite-type' cavities in its crystal structure. The structure of vladkrivovichevite is based on O – Pb derivative blocks with the interlayer occupied by Cl– anions and oxocentred OPb4Mn2 octahedra whose eight triangular faces are capped by triangular borate anions, BO33–.