Published online by Cambridge University Press: 02 January 2018
The crystal structure of hejtmanite, Ba2Mn4Ti2(Si2O7)2O2(OH)2F2, from Mbolve Hill, Mkushi River area, Central Province, Zambia (holotype material) has been refined on a twinned crystal to R 1 = 1.88% on the basis of 4539 [|F| > 4|F|] reflections. Hejtmanite is triclinic, C1̅, a = 10.716(2), b = 13.795(3), c = 11.778 (2) ,= 90.07(3),= 112.24(3),= 90.03(3), V = 1612(2) 3. Chemical analysis (electron microprobe) gives: Ta2O5 0.09, Nb2O5 1.27, ZrO2 0.65, TiO2 14.35, SiO2 23.13, BaO 26.68, SrO 0.19, FeO 11.28, MnO 15.12, Cs2O 0.05, K2O 0.33, F 3.82, H2Ocalc. 1.63, O = F 1.61, total 97.10 wt.%, where the H2O content was calculated from the crystal-structure refinement, with (OHF) = 4 apfu. The empirical formula, calculated on the basis of 20 (OF) anions, is of the form 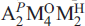 (Si2O7)2(XO)4(XP)2, Z=4: (Ba1.82K0.07 Sr0.02)Σ1.91(Mn2.33
(Si2O7)2(XO)4(XP)2, Z=4: (Ba1.82K0.07 Sr0.02)Σ1.91(Mn2.33 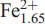 Zr0.04Mg0.03)Σ3.95(Ti1.88Nb0.10Zr0.02)Σ2(Si2.02O7)2O2[(OH)1.89 F0.11]Σ2F2. The crystal structure is a combination of a TS (Titanium Silicate) block and an I (intermediate) block. The TS block consists of HOH sheets (Hheteropolyhedral, Ooctahedral). The topology of the TS block is as in Group-II TS-block minerals: Ti ( Nb) = 2 apfu per (Si2O7)2 [as defined by Sokolova (2006)]. In the O sheet, five [6]MO sites are occupied mainly by Mn, less Fe2 and minor Zr and Mg, with <MOφ> = 2.198(φ = O,OH), ideally giving Mn4 apfu. In the H sheet, two [6]MH sites are occupied mainly by Ti, with <MHφ> = 1.962(φ = O,F), ideally giving Ti2 apfu; four [4] Si sites are occupied by Si, with < SiO> = 1.625 . The MH octahedra and Si2O7 groups constitute the H sheet. The two [12]Ba-dominant AP(1,2) sites, with <APφ> = 2.984(φ = O, F), ideally give Ba2 apfu. Two
Zr0.04Mg0.03)Σ3.95(Ti1.88Nb0.10Zr0.02)Σ2(Si2.02O7)2O2[(OH)1.89 F0.11]Σ2F2. The crystal structure is a combination of a TS (Titanium Silicate) block and an I (intermediate) block. The TS block consists of HOH sheets (Hheteropolyhedral, Ooctahedral). The topology of the TS block is as in Group-II TS-block minerals: Ti ( Nb) = 2 apfu per (Si2O7)2 [as defined by Sokolova (2006)]. In the O sheet, five [6]MO sites are occupied mainly by Mn, less Fe2 and minor Zr and Mg, with <MOφ> = 2.198(φ = O,OH), ideally giving Mn4 apfu. In the H sheet, two [6]MH sites are occupied mainly by Ti, with <MHφ> = 1.962(φ = O,F), ideally giving Ti2 apfu; four [4] Si sites are occupied by Si, with < SiO> = 1.625 . The MH octahedra and Si2O7 groups constitute the H sheet. The two [12]Ba-dominant AP(1,2) sites, with <APφ> = 2.984(φ = O, F), ideally give Ba2 apfu. Two 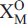 (1,2) and two
(1,2) and two 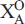 (1,2) sites are occupied by O atoms and OH groups with minor F, respectively, ideally giving (XO)4 = (
(1,2) sites are occupied by O atoms and OH groups with minor F, respectively, ideally giving (XO)4 = (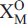 )2(
)2(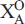 )2=O2(OH)2 pfu. Two
)2=O2(OH)2 pfu. Two 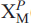 (1,2) sites are occupied by F, giving F2 apfu. TS blocks link via a layer of Ba atoms which constitute the I block. Simplified and end-member formulae of hejtmanite are Ba2(Mn,Fe2)4Ti2 (Si2O7)2O2(OH,F)2 F2 and Ba2Mn4Ti2(Si2O7)2O2(OH)2F2, Z = 4. Hejtmanite is a Mn-analogue of bafertisite, Ba2
(1,2) sites are occupied by F, giving F2 apfu. TS blocks link via a layer of Ba atoms which constitute the I block. Simplified and end-member formulae of hejtmanite are Ba2(Mn,Fe2)4Ti2 (Si2O7)2O2(OH,F)2 F2 and Ba2Mn4Ti2(Si2O7)2O2(OH)2F2, Z = 4. Hejtmanite is a Mn-analogue of bafertisite, Ba2 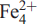 4 Ti2(Si2O7)2O2(OH)2F2.
4 Ti2(Si2O7)2O2(OH)2F2.