Article contents
Fluoroleakeite, NaNa2(Mg2Fe3+2Li)Si8O22F2, a new mineral of the amphibole group from the Verkhnee Espe deposit, Akjailyautas Mountains, Eastern Kazakhstan District, Kazakhstan: description and crystal structure
Published online by Cambridge University Press: 05 July 2018
Abstract
Fluoroleakeite, NaNa2(Mg2Fe3+2Li)Si8O22F2 is a new mineral of the amphibole group from the Verkhnee Espe deposit, Akjailyautas mountains, eastern Kazakhstan district, Kazakhstan. The granites and their host rocks have been intensely reworked by post-magmatic and host-rock fluids, resulting in intense recrystallization, enrichment in F, Li and rare elements, and replacement of primary biotite and sodic-calcic amphiboles by Li-bearing riebeckite, aegirine, astrophyllite and other sodic minerals including fluoroleakeite. Crystals are prismatic parallel to [001] with {100} and {110} faces and cleavage surfaces, and the prism direction is terminated by irregular fractures. Grains are up to 3 mm long, and occur as isolated crystals, as small aggregates, and as inclusions in cámaraite. Crystals are black with a very pale grey to colourless streak. Fluoroleakeite is brittle, has a Mohs hardness of 6 and a splintery fracture; it is non-fluorescent with perfect {110} cleavage, no observable parting, and has a calculated density of 3.245 g cm–3. In plane-polarized light, it is pleochroic, X = pale grey-green, Y = medium grey, Z = grey-brown; X^a = 14.1° (in β obtuse), Y ‖ b, Z^c = 75.9° (in β acute). Fluoroleakeite is biaxial negative, α = 1.663(2), β = 1.673(2), γ = 1.680(2); 2Vobs = 80.9(6)°, 2Vcalc = 79.4°
Fluoro-leakeite is monoclinic, space group C2/m, a = 9.8927(3), b = 17.9257(6), c = 5.2969(2) Å, β = 103.990(1)°, V = 905.7(1) Å3, Z = 2. The strongest ten X-ray diffraction lines in the powder pattern are [d in Å(I)(hkl)]: 2.718(100)(151), 8.434(40)(110), 4.464(30)(021), 3.405(30)(131), 3.137(20)(310), 2.541(20)(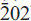 ), 2.166(20)(261), 2.325(15)(
), 2.166(20)(261), 2.325(15)(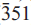 ), 2.275(15)(
), 2.275(15)(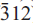 ) and 2.806(10)(330). Analysis by a combination of electron microprobe and crystal-structure refinement gives SiO2 53.34, Al2O3 0.62, TiO2 1.27, V2O3 0.05, Fe2O3 15.10, FeO 6.00, MnO 2.04, ZnO 0.18, MgO 6.40, CaO 0.13, Na2O 9.08, K2O 1.98, Li2O 1.10, F 3.33, H2Ocalc 0.16, sum 99.39 wt.%. The formula unit, calculated on the basis of 23 O, is A(Na0.64K0.38)(Na1.98Ca0.02)(Li0.66Mg1.42Fe0.752+Mn0.262+Zn0.02Fe1.693+V0.013+Ti0.144+Al0.03) (Si7.93Al0.07)O22(F1.57OH0.16O0.27). Crystal-structure refinement shows Li to be completely ordered at the M(3) site. Fluoroleakeite, ideally NaNa2(Mg2Fe23+Li)Si8O22F2, is related to end-member leakeite, NaNa2(Mg2Fe23+Li)Si8O22(OH)2 by the substitution F → (OH).
) and 2.806(10)(330). Analysis by a combination of electron microprobe and crystal-structure refinement gives SiO2 53.34, Al2O3 0.62, TiO2 1.27, V2O3 0.05, Fe2O3 15.10, FeO 6.00, MnO 2.04, ZnO 0.18, MgO 6.40, CaO 0.13, Na2O 9.08, K2O 1.98, Li2O 1.10, F 3.33, H2Ocalc 0.16, sum 99.39 wt.%. The formula unit, calculated on the basis of 23 O, is A(Na0.64K0.38)(Na1.98Ca0.02)(Li0.66Mg1.42Fe0.752+Mn0.262+Zn0.02Fe1.693+V0.013+Ti0.144+Al0.03) (Si7.93Al0.07)O22(F1.57OH0.16O0.27). Crystal-structure refinement shows Li to be completely ordered at the M(3) site. Fluoroleakeite, ideally NaNa2(Mg2Fe23+Li)Si8O22F2, is related to end-member leakeite, NaNa2(Mg2Fe23+Li)Si8O22(OH)2 by the substitution F → (OH).
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2010
References
- 6
- Cited by


