Article contents
Barikaite, Pb10Ag3(Sb8As11)Σ19S40, a new member of the sartorite homologous series
Published online by Cambridge University Press: 05 July 2018
Abstract
Barikaite, ideally Pb10Ag3(Sb8As11)Σ19S40, is a new mineral species from the Barika Au-Ag deposit, Azarbaijan Province, western Iran. It was formed in fractures developed in silica bands situated in massive banded pyrite and baryte ores. These fractures house veinlets that contain a number of Ag-As-Sb-Pb-rich sulfosalts, tetrahedrite-tennantite, realgar, pyrite and electrum. Barikaite appears as inclusions in guettardite. The mineral is opaque, greyish black with a metallic lustre; it is brittle without any discernible cleavage. In reflected light barikaite is greyish white, pleochroism is distinct, white to dark grey. Internal reflections are absent. In crossed polars, anisotropism is distinct with rotation tints in shades of grey. The reflectance data (%, in air) are: 37.0, 39.3 at 470 nm, 34.1, 36.9 at 546 nm, 33.1, 36.2 at 589 nm and 31.3, 34.1 at 650 nm. The Mohs hardness is 3–3½, microhardness VHN50 exhibits the range 192 – 212, with a mean value of 200 kg mm–2. The average results of five electron-microprobe analyses in a grain are (in wt.%): Pb 35.77(33), Ag 5.8(1), Tl 0.15(08), Sb 18.33(09), As 15.64(16), S 24.00(15), total 99.69(10) wt.%, corresponding to Pb9.31Ag2.90Tl0.04(Sb8.12As11.26)Σ19.36S40.37 (on the basis of 32Me + 40S = 72 a.p.f.u.). The simplified formula, Pb10Ag3(Sb8As11)Σ19S40, is in accordance with the results of a crystal-structure analysis, and requires Pb 37.89, Ag 5.91, Sb 17.79, As 15.05 and S 23.42 (wt.%). The variation of chemical composition is minor, the empirical formula ranging from Pb10.39Ag2.32Tl0.02Sb7.52As11.27S40.49 to Pb9.24Ag2.93Tl0.04Sb8.13As11.35S40.31. Barikaite has monoclinic symmetry, space group P21/n and unit-cell parameters a 8.5325(7) Å, b 8.0749(7) Å, c 24.828(2) Å, and b 99.077(6)o, Z = 1. Calculated density for the empirical formula is 5.34 (g cm–3). The strongest eight lines in the (calculated) powder-diffraction pattern [d in Å(I)(hkl)] are: 3.835(63)(022), 3.646(100)(016), 3.441(60)(212), 3.408(62)(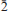 14), 2.972(66)(
14), 2.972(66)(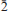 16), 2.769(91)(222), 2.752(78)(
16), 2.769(91)(222), 2.752(78)(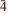 24) and 2.133(54)(402). Barikaite is the N = 4 member of the sartorite homologous series with a near-equal role of As and Sb, which have an ordered distribution pattern in the structure. It is a close homeotype of rathite and more distantly related to dufrénoysite (both distinct, pure arsenian N = 4 members) and it completes the spectrum of Sb-rich members of the sartorite homologous series. The new mineral and its name have been approved by the IMA-CNMNC (IMA 2012-055).
24) and 2.133(54)(402). Barikaite is the N = 4 member of the sartorite homologous series with a near-equal role of As and Sb, which have an ordered distribution pattern in the structure. It is a close homeotype of rathite and more distantly related to dufrénoysite (both distinct, pure arsenian N = 4 members) and it completes the spectrum of Sb-rich members of the sartorite homologous series. The new mineral and its name have been approved by the IMA-CNMNC (IMA 2012-055).
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2013
References
- 8
- Cited by


