Article contents
Aluminopyracmonite, (NH4)3Al(SO4)3, a new ammonium aluminium sulfate from La Fossa crater, Vulcano, Aeolian Islands, Italy
Published online by Cambridge University Press: 05 July 2018
Abstract
The new mineral aluminopyracmonite, ideally (NH4)3Al(SO4)3, was found in a medium-temperature (∼250°C) intracrater active fumarole at La Fossa crater, Vulcano, Aeolian Islands, Sicily, Italy. It occurs on a pyroclastic breccia as colourless to white prismatic crystals up to 0.2 mm long, in association with adranosite, mascagnite, alunite and salammoniac. The mineral is identical to the synthetic compound (NH4)3Al(SO4)3. It is trigonal, space group: R (no. 148) with a = 15.0324(8), c = 8.8776(5) 5, V = 1737.3(2) Å3 and Z = 6. The six strongest reflections in the X-ray powder diffraction pattern are: [dobs in Å (I)(hkl)] 3.336(100)(131), 7.469(62)(1 1 0), 3.288(60)(122), 4.289(45)(
(no. 148) with a = 15.0324(8), c = 8.8776(5) 5, V = 1737.3(2) Å3 and Z = 6. The six strongest reflections in the X-ray powder diffraction pattern are: [dobs in Å (I)(hkl)] 3.336(100)(131), 7.469(62)(1 1 0), 3.288(60)(122), 4.289(45)(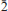 31), 2.824(29)(
31), 2.824(29)( 51), 4.187(27) (012). The empirical formula based on 12 anions is [(NH4)2.89K0.10]Σ 2.99(Al1.18Fe0.01)Σ 1.19S2.91O12, and the simplified formula (NH4,K)3Al(SO4)3. The measured density is 2.12(1) g/cm3, calculated density 2.143 g/cm3. The mineral is uniaxial(–) with ω = 1.545(3) and ε = 1.532(3) (λ = 589 nm). Using single-crystal diffraction data, the structure was refined to a final R(F) = 0.0258 for 998 independent observed reflections [I > 2σ(I)]. In spite of having unitcell parameters comparable with those of pyracmonite, the two minerals are not isostructural; the difference is related to a disordered conformation of the sulfate anions about the two independent Al3+ ions in aluminopyracmonite.
51), 4.187(27) (012). The empirical formula based on 12 anions is [(NH4)2.89K0.10]Σ 2.99(Al1.18Fe0.01)Σ 1.19S2.91O12, and the simplified formula (NH4,K)3Al(SO4)3. The measured density is 2.12(1) g/cm3, calculated density 2.143 g/cm3. The mineral is uniaxial(–) with ω = 1.545(3) and ε = 1.532(3) (λ = 589 nm). Using single-crystal diffraction data, the structure was refined to a final R(F) = 0.0258 for 998 independent observed reflections [I > 2σ(I)]. In spite of having unitcell parameters comparable with those of pyracmonite, the two minerals are not isostructural; the difference is related to a disordered conformation of the sulfate anions about the two independent Al3+ ions in aluminopyracmonite.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2013
References
- 9
- Cited by


