Introduction
Between the end of World War II and the early 1950s, the incidence of poliomyelitis increased steadily on both sides of the Atlantic, and gave rise to new epidemics in European countries with considerable time differences from northern areas to those in the south, where it occurred later.1 This north–south gradient explained in part the different rhythms of Europe in responding to this social and health problem.2 In the United States, the epidemics afflicting young children each summer rapidly became a major public health issue, occupying an increasing place in the media and in health administration meetings year after year. However, in France, polio was certainly a worrying issue but considered less pressing than infant mortality from diphtheria, tuberculosis or complications from eruptive fevers.3 With a political context and a mobilisation favourable to the fight against the disease, the two main polio vaccines were developed in the United States: the injected polio vaccine (IPV) developed by Jonas Salk (1914–95) and the oral polio vaccine (OPV) by Albert Sabin (1906–93). European scientists were also working on this topic during the 1950s. One of these was Pierre Lépine (1901–89), a French biologist and physician working at the Institut Pasteur of Paris, who also developed an effective vaccine that was technologically close to the Salk’s IPV, but not totally identical, in order to be safer and to avoid problems like the Cutter incident.4 However, the Salk vaccine quickly supplanted the Lépine vaccine throughout the world.
There is a rich literature regarding polio control and vaccination in the United States.5 The disease has had such an impact on the country that it also appears in popular literature.6 Work concerning many European countries has also been carried out these last years.7 But paradoxically, very few studies have been carried out on the French case.8 Perhaps the smaller impact of the disease and of the movements of poliomyelitis victims in France (compared to the United States, for example) is responsible for this shortcoming. Or the delay in controlling the disease by vaccination, together with difficulties for its generalisation, did not fuel enthusiasm for an analysis of the French case. The development of effective vaccines to fight a disease in no way presumes how they should be used; let alone how they are ultimately used.
This article analyses the polio control strategies and policies implemented by France from the mid-1950s (development of the Lépine vaccine, the first effective polio vaccine used in the country) to the end of the 1960s when strategies and policies had been stabilised, especially with the adoption of mandatory polio vaccination. In order to carry out this analysis, the article examines the role played by two main actors and institutions: Pierre Lépine and the Institut Pasteur of Paris, one of the reference bacteriological research centres from the nineteenth century onwards, and Charles Mérieux (1907–2001) and the Institut Mérieux of Lyon, a private pharmaceutical laboratory and one of the two producers of polio vaccines in France, along with the Institut Pasteur. In addition, the influence of foreign research stays of leading French scientists involved in the process is also studied.
This work, based on original archives from the Pasteur and Mérieux institutes, as well as the Symposia of the European Association Against Poliomyelitis (EAP), a selection of French scientific journals, official documents of the World Health Organization (WHO) and oral sources,9 focuses on the different actions and manoeuvres implemented by the two institutes and their main representatives, so as to mobilise the French health authorities against polio. If the two main protagonists worked together, they each had different influences, at different times with the aim to raise awareness of the fight against the disease. Moreover, we examine the foreign scientific relations of the key French actors who were decisive in the development and production of the polio vaccines and their application, but especially the strategies and trajectories through which the value of these vaccines was highlighted. These strategies and trajectories were regularly modified according to the obstacles and complications encountered.
The study should contribute to understanding the processes of choice of polio vaccines at the level of national institutions. The French case shows how the driving force of the development of its own vaccine was to get a safer and effective IPV than Salk’s and not urgency, as it was the case of the United States.10 It also represents an example of how mass production and administration of poliomyelitis vaccine in France was the result of the relationship established between a public research institute, the Institut Pasteur, which had had the monopoly of serum and vaccines production, and a private pharmaceutical laboratory, the Institut Mérieux, at a moment when the French pharmaceutical industry was demanding more development, as in the United States, Germany and Switzerland.11 Although the history of the polio vaccines and vaccination has been extensively studied, the Pasteur and Mérieux institutes’ attempt to mobilise against the disease in France merits closer examination in the context of the time, as well as in the light of more recent works. This study also sheds new light on the history of vaccination politics. To be sure, as mentioned above, there is no shortage of relevant works published over recent years. Studies typically explore how vaccines were developed, produced and used over a period of time and in a particular context. They rarely follow the construction of debates – and their evolution over time – within scientific and political networks, a fortiori by actors directly involved in vaccine production and administration. By showing the manoeuvres and trajectories taken to support polio vaccination, this study provides deeper insight into the political and scientific decisions health authorities take relating to vaccination, and into the trade-offs between risks of infection, safety, cultural acceptance and resource availability.
Lépine, the Institut Pasteur and the French polio vaccine
With the importance that polio assumed in the United States, the mobilisation of the population, and the extensive action of the management and the financing of research by the National Foundation for Infantile Paralysis, Jonas Salk developed an effective inactivated/killed virus vaccine in 1954, and mass application began on 12 April 1955.12 Only a few days later, the research begun in different European laboratories allowed for the announcement of the first European vaccine, also inactivated, and developed by the Swedish Sven Gard (1905–98).13 However, hopes arising from Salk’s and Gard’s vaccines were dashed when the Cutter incident took place at the end of April 1955. Of the children who received the vaccine in the United States, seventy-nine developed paralytic poliomyelitis, and of these, eleven died from polio. The responsibility for the accident was attributed to the Cutter Laboratories, one of several companies authorised to produce Salk’s polio vaccine. Some lots of the Cutter vaccine contained live poliovirus in what was supposed to be an inactivated-virus vaccine.14 This event delayed the implementation of mass immunisation campaigns on both sides of the Atlantic, and led to the development and introduction of important security measures in the production and administration of the vaccines, which were promoted by the WHO together with the EAP. The WHO, through an international Technical Committee on Poliomyelitis Vaccination, revised production procedures during 1955 and changed them five times before establishing and publishing in December 1955 the final basic recommendations to be followed for the production of a safer and more effective vaccine, known as the Minimal Requirements.15 The EAP, created in 1951, was particularly relevant for the European countries’ search for a procedure of their own, through its symposia, which played an important role in the circulation of scientific knowledge and practices about the disease, the epidemiological situation and the development and implementation of immunisation in Europe.
Although the EAP initially sought to create a single safer and more appropriate path to combat polio for all countries, the European response differed from country to country, because of its adaptation to the specific circumstances of each country.16 Strategies as well as time schedules differed, the starting point of vaccination ranging from 1955 to the beginning of the 1960s. While Denmark and Finland started in 1955, France, Iceland, Norway, Belgium, the United Kingdom, Federal Germany and Switzerland did so in 1956, and the majority of other European countries (including Sweden) in 1957.17 The type and origin of vaccines applied also differed. The existence of bacteriological laboratories with a long tradition of research favoured local production of their own vaccine and early immunisation,18 although some practical problems arose, due to difficulties in producing a sufficient quantity of vaccine to achieve immunisation. These countries had to buy the vaccine from foreign pharmaceutical laboratories and/or delay the national campaign. One of the latter cases was Sweden, where Sven Gard developed an inactivated vaccine at the same time as Salk, although the first national immunisation campaign had to be delayed until 1957 because of the limited production capacity of his laboratory at the Karolinska Institute (Stockholm). The Swedish vaccine gave better results (after 1961, all the sporadic cases declared were imported) and had a different composition. It contained a type I virus strain,19 which was less virulent than the Mahoney strain included in the Salk vaccine, and was offered free of charge.
In France, Pierre Lépine, physician and director of the Virus Department at the Institut Pasteur in Paris, developed at the beginning of the 1950s a vaccine technologically close to that developed by Salk, which was used for the first time in 1956. However, the American and French vaccines differed in a number of points, the most important of which were the use of African monkeys20 (instead of Asian monkeys for the American vaccine); the double inactivation procedure (with formaldehyde and propiolactone), and a preponderance – in the Lépine vaccine – of the type I antigen, which remained the most common in Europe. The development of the French vaccine was the result of a long tradition of research by the Institut Pasteur on poliomyelitis – epidemiology and virus – started by the works of Constantin Levaditi (1874–1953), in collaboration with Karl Landsteiner (1868–1943),21 at the end of the second decade of the twentieth century.22 Levaditi, a Romanian physician and microbiologist, introduced Lépine to the study of the poliovirus focused on different aspects, which were important for achieving the aim of producing his own vaccine against poliomyelitis. One of these was the study of the presence of poliovirus in sewage and its impact on disease diffusion, as well as the impact of a useful method – chloration or hot inactivation – for their elimination.23 Later, this knowledge, in collaboration with his technical assistant Valentine Emma Sautter, inspired the development of a double inactivation procedure (with formaldehyde and propiolactone) of his vaccine, making it safer than Salk’s vaccine without losing the capacity of producing immunity.24
Lépine, the first President of the EAP from its creation in 1951, also worked on the culture of poliovirus and the selection of different safer strains for producing a new vaccine. Very important to this task was his visit to the laboratory of Jonas Salk and his relationship with Canadian researchers, particularly from the Connaught Laboratory and the Institute of Microbiology and Hygiene of the University of Montreal. In these institutions, Lépine carried out different scientific visits and courses and had a laboratory for working on the adaptation of John Franklin Enders’ (1897–1985) techniques of in vitro culture of poliovirus, using the strains Salk supplied to the Canadian researchers.25 Together with the Canadian professor Vytautas Pavilanis (1920–2006),26 Lépine set up a modified procedure for the production of a vaccine, with more complete extraction of the virus in order to get more powerful immunity, published in 1952.27 Finally, Lépine’s vaccine included a strain of poliovirus type I different to the Mahoney used by Salk for his vaccine, IP 1352, together with IP 1526 for type II, and IP 1226 for type III of poliovirus.28
Pierre Lépine had trouble achieving mass production of his vaccine in the Institut Pasteur. He needed the construction of a new building, more equipment and sufficient specialised personnel, which he requested to the French Government and the National Center for Scientific Research (CNRS) in 1953,29 having previously acquired adequate preparation during his research visits to the United States and Canada, mentioned above. However, the French Government and the CNRS paid no attention to his request,30 and the production and commercialisation of Lépine’s vaccine were delayed. He undertook considerable national and international activities during 1954 in order to overcome the obstacles to his project. He stopped the classification of poliovirus in his department at the beginning of the summer of 1954, and explained the reasons for this decision. At the same time, he started another scientific visit to Canada and threatened that he would produce his vaccine in the Connaught laboratory and send it to France, thanks to the Institut Mérieux. Moreover, in 1954, probably making use of his membership of the Expert Committee on Poliomyelitis of the WHO, he obtained the support of Anthony Payne,31 and his laboratory was included in the network of WHO poliomyelitis regional centres, being designated as the poliomyelitis centre for the European Region of the WHO in November 1954.32 This may be connected to the change of attitude of the director of the Institut Pasteur to the demands of Lépine and the announcement by the French newspaper L’Aurore, that the production of Lépine’s inactivated vaccine could start in Paris.33
In January 1955, Lépine confirmed that, during his latest stay in Canada, he had perfected a vaccine at the experimental stage.34 However, it was still not possible to produce it in a large quantity, and in May 1955, he therefore declared himself against the mass use of an inactivated vaccine in France, although he was in favour of using it within a broad serological survey, in collaboration with the paediatrician and president of the National Institute of Hygiene, Robert Debré (1882–1978), and his laboratory in the Hôpital Necker–Enfants malades in Paris. He also proposed that this survey should be backed and financed by the French Ministry of Health.35 Lépine’s plan was to have his vaccine available and on the market by 1 January 1956, despite his laboratory’s limited production capacity.36 Lépine’s vaccine reached French pharmacies in late March 1956, and the first twelve public vaccination centres (PVCs), created expressly to administer this vaccine, some months later. According to Gérard Orth (1936–), a contemporary of Lépine in the Virus Department of the Institut Pasteur, the first experimental vaccines were given to volunteers on 1 June 1956, in the institute’s Virus Department,37 and the first tests and serological studies were carried out on patients of the Pasteur Hospital in Paris.38 Lépine did not have the capacity to produce his vaccine on a large scale until 1957.39
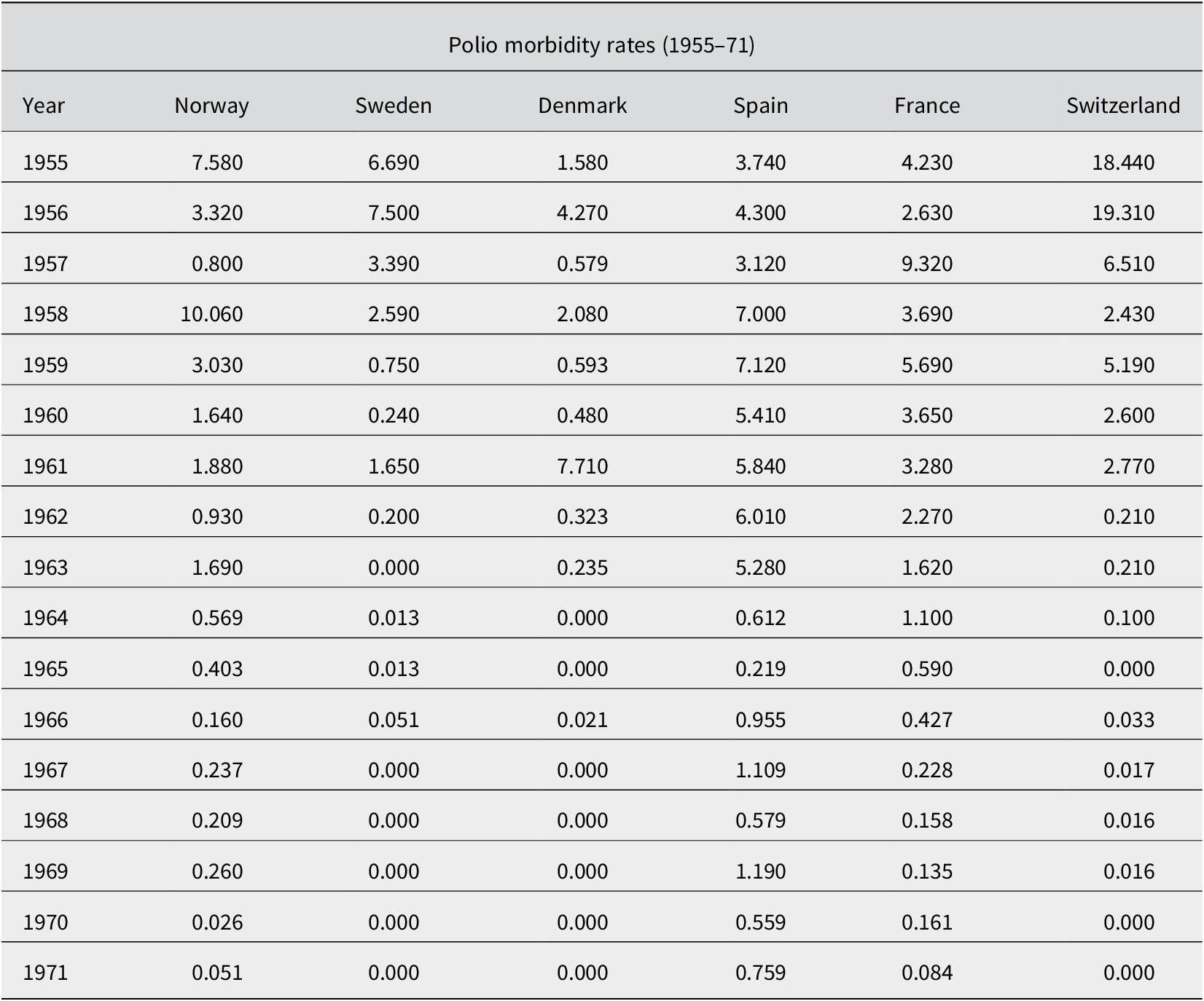
Until 1957, vaccination against polio in France had not been conceived as a mass vaccination campaign, nor had it been considered necessary to develop a nationwide vaccination program.40 As we can see in Table 1, French polio morbidity rates were lower than the majority of countries of the European Region of the WHO, before the existence of the first vaccine against poliomyelitis. However, in 1957, France reached its highest level of incidence of polio, with a morbidity rate of 9.32 per 100 000 inhabitants (Table 2), a situation which, due to the limited extent of the trials of the Lépine vaccine, restricted access of the population, and probably the lack of sufficient information on its effectiveness, greatly worried the Ministry of Public Health, which asked about the number of doses available and the real production capacity of the Institut Pasteur.41 Nor can we rule out the impact on the Ministry’s concerns of the debates and conclusions of the EAP, favourable to the generalisation of vaccination in European countries.42 Consequently, in July 1957, the Ministry obliged Lépine and Jacques Tréfouël, then director of the institute, to initiate an emergency plan to increase significantly the quantity of vaccine available. It was intended to go from a production of 14 litres per week of vaccine to 500 litres per day.43 Faced with this demand from the health authorities, and the fact that industrial scale production of the vaccine could not be carried out in the Institut Pasteur as Lépine had originally envisioned, the institute undertook the construction of a specific laboratory in the Institut Pasteur in Garches, in Villeneuve–l’Étang,44 which was inaugurated on 29 January 1958 and was headed by the veterinarian, Dr Cheyroux.45
Table 1. Polio morbidity rates of countries of the WHO European Region (1947–54). Own elaboration with data from Mathieu-Jean Freyche and Johannes Nielsen, ‘Incidencia de la poliomielitis desde 1920’, Boletín de la Oficina Sanitaria Panamericana, 40, 1 (1956), 15–52; World Health Organization, Poliomyelitis (Geneva: WHO, 1955), 59–105

Table 2. Polio morbidity rates (1955–71). Own elaboration with data from Enrique Nájera et al., ‘Análisis epidemiológico de la situación actual de la poliomielitis en España’, Revista de Sanidad e Higiene Pública, 49 (1975), 953–1025
Industrial scale production of the Lépine vaccine
During the First International Congress on Biological Standardization, organised by the Institut Mérieux in Lyon in 1956, the differences between the Salk and Lépine vaccines had caught the attention of the physician and director of the Institut Mérieux, Charles Mérieux, the son of the microbiologist Marcel Mérieux, founder of the institute of the same name in 1897. He was particularly struck by the method of inactivation of the Lépine vaccine (in two stages, the first with formaldehyde for 48 hours, then with propiolactone for 2 hours at low temperature, completing the inactivation in just 72 hours, compared to the 9–12 days of Salk’s and 96 hours of Gard’s vaccine).46 After this Congress, the Institut Mérieux obtained a licence from the Institut Pasteur for the production of the Lépine vaccine. However, at the request of its director Jacques Tréfouël, industrial production of the vaccine was not yet envisaged. The extremely low level of sales did not require the increase in production initially provided by the Institut Pasteur.47 Tréfouël’s position, in favour of keeping the production of the Lépine vaccine by the Institut Pasteur instead of permitting the participation of the pharmaceutical laboratory, was consistent with the maintenance of the Institut Pasteur’s monopoly in the production of vaccines in France.
However, in 1958, after the important increase of polio morbidity rates in 1957 (Table 2), an agreement was established between the Institut Mérieux and the Institut Pasteur for the production and distribution of the Lépine vaccine: the Lyon institute was to supply a quarter of the vaccines intended for vaccinations.48 Initially, the Lyon laboratories were not specifically equipped for a large production of polio vaccine. However, faced with the upsurge in cases in France and the worldwide generalisation of polio vaccination, the Institut Mérieux decided to set up specialised laboratories for the industrial production of the vaccine. The first installations were ready in mid-January,49 and at the end of the month, Lépine had contacted the Lyon teams for the technical procedures to be followed.50 However, industrial production of the polio vaccine was not expected to peak until September, when more vaccine would be needed in July and August. In fact, polio epidemics raged seasonally in summer before the generalisation of vaccination coverage. The Minister of Health had at one time been considering importing the Salk vaccine from the United States,51 allowing immediate troubleshooting. However, according to Charles Mérieux, such a manoeuvre: ‘would introduce the Salk vaccine into our country, the essential difference of which from the Lépine vaccine lies in the type of strain Nº I’.52 Mérieux skilfully made sure to keep a French production of the polio vaccine with this type of discourse, which was in line with what the EAP had promoted since its first Symposium.53
From October 1958, a first polio vaccination campaign was organised by the Ministry of Public Health.54 Vaccinations were to be carried out at PVCs. These centres were supplied with vaccine via orders from the Departmental Directorates of Health with centralised orders at the Institut Pasteur. The distribution of these deliveries – between the Lyon institute and the Paris institute – was managed by the Institut Pasteur. Organising a nationwide immunisation campaign required specific information on the regular availability of the vaccine for the PVCs. Advance registrations for vaccinations could provide valid information on the vaccine needs of these centres. However, the optional nature of vaccination made the activity of the centres essentially dependent on the actual demands of the population. Indeed, polio vaccination was not yet compulsory. At that time in France, only vaccines against smallpox, diphtheria, tetanus and tuberculosis were mandatory.55 This non-compulsory nature consequently made it difficult to quantify even approximately and in the very long term the real vaccine needs of the PVCs. The Director General of Public Health, Dr Aujaleu, therefore requested the Institut Mérieux to reserve part of the production for the public sector, and more particularly for this vaccination campaign.56 With the industrial improvements carried out a few months earlier, the Institut Mérieux could reach the weekly rate of 100 000 doses, or even increase this production if necessary.57 Taking into account the agreement signed with the Institut Pasteur (supplying a quarter of the production), the Lyon institute estimated that a weekly supply of 20 000 ampoules to the PVCs was sufficient to satisfy the expectations of the Director General of Public Health. In addition, the Institut Mérieux had introduced an important avenue for the use of the vaccine: it seemed desirable that costs should be reimbursed by the Social Security. In the pharmaceutical circuit, reimbursement could encourage physicians and pharmacists to help roll out the vaccination campaign.58 However, the industrial production capacity of tens of thousands of doses of vaccine per week by Institut Mérieux encountered a problem: the flow of vaccines. Thus, the vaccination campaign at the end of 1958 did not have the expected success: people’s demands were very limited, probably because the PVCs did not really have funds available for the purchase of vaccines, people were not very familiar with these new public centres and preferred their doctors; and the reimbursement of this vaccination by the Social Security was still not recorded. The Lyon institute had asked the Ministry for help to intensify the propaganda effort concerning the polio vaccination and to obtain reimbursement (at least partially).59 These strategies could allow an increase in the demand for the vaccine and the profits of the Institut Mérieux by reducing the flow of vaccines.
Several months after these requests, the Institut Mérieux had still not received a favourable response. In letters to Dr Aujaleu, Charles Mérieux pointed out that the agreement for the distribution of vaccines between Lyon and Paris was not being respected. At equal production, Lyon’s output was extremely low. The Lyon laboratories therefore found themselves with a large stock of vaccine that was difficult to sell. Faced with this problem, the Lyon institute threatened the Ministry to slow down or even completely stop the production of the vaccine. This threat was also accompanied by a well-aimed barb: ‘but I would like to be sure beforehand not to disappoint the Ministry, which, a few months ago, found our stocks insufficient’.60 If the Ministry wished to keep two French production centres for the Lépine vaccine, it had to agree to verify the equitable flow of stocks between Paris and Lyon. It seemed difficult for a private laboratory – with modest visibility from a national point of view – to fight commercially against the Institut Pasteur, which enjoyed free publicity in the press, radio and television, especially with an identical product, under licence from the Paris institute. To emphasise the economic aspect of the production, which was the responsibility of the Lyon institute, Charles Mérieux recalled that: ‘we charge the same prices as the Institut Pasteur, while having to pay a royalty for it, reimburse the National Credit, pay 20% of taxes, not to mention other taxes’.61
In 1959, according to the health authorities, there were already 550 PVCs open throughout the country, administering the vaccine free of charge to the target groups, mainly subjects from 1 to 20 years of age, and coverage in the older age groups began to increase. However, in September 1959, the representative of the French Ministry of Public Health reported at the VIe Symposium of the EAP, held in Munich, that out of the 1 500 000 people vaccinated in France so far, only 300 000 had been vaccinated in the 550 government centres,62 despite the fact that the polio vaccination program in France was already being publicised by radio and in the press, both at departmental and national levels, in order to encourage the participation of the population.63 In 1958, polio morbidity decreased in France after the introduction to the calendar of a three-dose scheme of vaccine, to which Lépine recommended adding a fourth booster dose.64 However, in 1959, France continued to suffer severely from the disease, with morbidity rate of 5.69 cases per 100 000 inhabitants (Table 2), and cases of paralytic poliomyelitis remained numerous (2 564 cases in 1959). There was a significant number of deaths from the disease during these first years of the introduction of vaccination in the country (8.4% in 1958 and 1959), due to the fact that vaccination coverage did not reach adequate levels to be effective. In fact, the coverage was 4% (under 4 years), 6.5% (5–14 years) and 2% (15–20 years) in 1958, and 8% (under 4 years), 14% (5–14 years) and 5% (15–20 years) in 1959.65
In parallel with the requests made to the Ministry, the Lyon institute was trying for its part to call on the National Syndicate of Physicians in order to further mobilise the medical community about polio prophylaxis.66 This approach was part of a draft collective agreement on preventive medicine currently being negotiated between the National Syndicate of Physicians and the Social Security. This therefore presented a unique opportunity to include the reimbursement of the polio vaccine in the negotiations. The request was based on the essential role that the family physician should play in the immunisation schedule.67 Immunisation of children seemed more easily achievable in the family physician setting rather than in the PVCs. This strategy seemed to be more consistent with the French tradition from 1938, when the antidiphtheria vaccination became compulsory.68 Furthermore, this argument was supported by revealing figures: 300 000 polio vaccinations in the PVCs against 1 175 000 vaccinations by family physicians, especially since the reimbursement of this vaccination was still pending, following opposition from the Ministry of Finance despite the favourable opinion of the Public Health Ministry and the Ministry of Labour.69 With the shortcomings of the Public Health Ministry, without credits, and the ensuing failure to supply and use of PVCs, the Institut Mérieux began to look to new allies to mobilise against polio. The ‘normal’ circuit of the physician and the pharmacist was able to constitute a more flexible and more economical form in the fight against the disease. At the time of this alternative in 1959, as we had indicated, France was behind schedule in polio vaccination. In Europe, only Spain had a lower rate,70 because of the lack of scientific and economic resources as well of the political decision of the Franco regime.71
A technical advance would also serve to facilitate the prophylaxis of polio: associated vaccinations. The Institut Mérieux had developed a so-called ampoule–syringe model.72 The latter could facilitate the practice of compulsory vaccinations (diphtheria and tetanus) by incorporating the polio valence in the D.T. or D.T. Coq (for pertussis) valences. Although Mérieux did not mention it, this new strategy could promote compulsory vaccination against poliomyelitis. The Institut Mérieux had once again called on the Director General of Public Health, Dr Aujaleu, to encourage the Departmental Directors of Health to promote and use this new model. A persistence of polio in the early winter of 1959 in France – linked to the low vaccination coverage of the disease – would certainly cause intensive use of the Lépine vaccine. The practice of associated vaccinations, in particular D.T. Polio, would make it possible to carry out economically compulsory vaccinations and especially booster injections.73 The strategy deployed by the Institut Mérieux, which was also used by the Netherlands,74 was brilliant. In order to increase polio vaccinations, it had proposed incorporating the polio antigenic valence into the mandatory D.T. vaccines. This manoeuvre could kill two (or even three) birds with one stone: mandatory vaccinations favoured polio vaccination; the polio vaccination made it possible to recall the compulsory vaccinations and the booster vaccinations (low vaccination coverage at that time); the combined vaccination limited the number of injections, protecting against diphtheria, tetanus and polio in one shot.
Introduction of another vaccine and the path to compulsory polio vaccination
The appearance of Sabin’s oral vaccine in 1957 and the announcement of the good results of its use in the Ve Symposium of the EAP (Madrid 1958) and, above all, in the VIe Symposium of the EAP (Munich 1959), the Report of the WHO committee of experts on poliomyelitis (June 1960) and the IVe International Poliomyelitis Congress (July 1960) transformed the fight against the disease by offering a new type of vaccine, easier to administer, but which, among other things, also required a reorganisation of the WHO’s network of regional centres.75 This transformation and the new possibilities that it offered were also noticed by Charles Mérieux. In October 1960, the Institut Mérieux applied to Albert Sabin for authorisation to produce the live attenuated polio vaccine or OPV. Sabin agreed under certain conditions – mostly technical – without asking for financial compensation of any kind in return.76 The Lyon institute asked Sabin if he had sent any strains of the virus to the Institut Pasteur. He replied that he had sent some to André Lwoff and Robert Debré,77 and later also to Pierre Lépine.78 The Institut Mérieux was perhaps trying to find out whether the Institut Pasteur would be a competitor in the production of the OPV, so as not to relive the problem that the Lyon institute had encountered with the Lépine vaccine.79 Albert Sabin was invited to Lyon in May 1961 for a symposium on the live attenuated virus vaccine, with the main European producers and controllers.80 This meeting was placed under the patronage of the Microbiological Standardization Section of the International Association of Microbiological Societies.81 It had been promoted by Charles Mérieux, in view of the scarce biological standardisation of these products, which enormously complicated the quality control of serums and vaccines and made comparison between them difficult, causing serious problems for import and export, and hindered international cooperation in worldwide prevention campaigns.82
The Institut Mérieux was equipped for the production and control of OPV, with specialised teams and premises. The efforts put in place for the production of the OPV were mainly financed by the sales of the Lépine vaccine.83 By then, Roger Sohier, director of the Virological Section of the National Control Laboratory based in Lyon, persuaded by the success of the campaigns implemented in different parts of the world, notably the USSR, was already carrying out small-scale trials in his laboratory at Lyon University with a Cox-Lederle trivalent oral vaccine and the Sabin vaccine, and pointed out the advantages of live rather than inactivated vaccines.84 Because of the position he held, Sohier’s opinion was very important and could help or hinder the interests of the Institut Mérieux. The possibility of the availability of attenuated vaccines, and the prominence of the Sabin vaccine, triggered an important debate on the pros and cons of each type of vaccine, which also took place in the Symposia of the EAP,85 and even led to smear campaigns from inactivated vaccines against oral vaccines, and vice versa. They were based on some of the weaknesses they had, such as contamination with the SV40 virus in the Salk vaccine.
Although the Institut Mérieux was also authorised to produce the Sabin vaccine, on 2 October 1961, Charles Mérieux recommended that Lépine should publish in the Academy of Medicine that the method used to make the vaccine suppressed the SV40 virus.86 The inactivation procedure of the poliovirus in the Lépine vaccine inactivated other viruses which would normally contaminate the monkey kidney cells.87 Mérieux justified this publication in order to mark a clear difference from other inactivated vaccines which did have that problem,88 and because, in France, there was: ‘pressure in favour of the Sabin vaccine and reservations about the Lépine vaccine, on the pretext that there was nothing to prove the absence of the famous simian virus’.89 Like Charles Mérieux himself, the strategy of scientific distribution which asked Lépine to point out the advantages of the French vaccine was at that time the key point by which he hoped that the new Ministry would back the vaccinations. This decision would be in the interests of the Institut Mérieux and of French public health policy, because it could improve the protection of the target population against polio (under 20 years): this was still low in the late 1960s, when vaccine coverage of children under 4 had only reached 25% of the total, 42.5% of those between 5 and 14, and only 15% of the 15–20 age group.90
The Institut Mérieux seemed to play on the controversy of the SV40 virus to support the quality of the French vaccine, which did not have this contamination problem.91 As Charles Mérieux said: ‘apart from the English at Wellcome, no one in Europe has worked so conscientiously: I am a little scandalised to see how certain European laboratories ‘camouflage’ the famous SV40 with laboratory tricks’.92 According to Mérieux, this lead: ‘should encourage our authorities in France to slow down the authorisation of the Sabin, but their only adviser, Mr. Sohier, will have to be convinced of it’.93 A strange reflection on Mérieux’s part, when he himself produced and sold the Sabin vaccine. But perhaps he understood that it would favour the use of the Lépine vaccine that he was producing himself, given that the authorisation of the sale of OPV was delayed in France.94
In parallel with the controversy over the SV40 virus, French legislation on polio vaccination had advanced. Lépine also played a part in this: in February 1962, in the National Academy of Medicine, he advocated that polio vaccination in France should be compulsory or, at least, free of charge.95 This proposal must be understood in light of the fact that the polio morbidity rate in 1962 was 2.27 per 100 000, still higher than in Denmark, Sweden, Norway and Switzerland (Table 2).96 Only Spain had higher morbidity rates (6.01 per 100 000) than France. A draft bill establishing compulsory and free polio vaccination was tabled in the Senate on 29 January 1962 (N°155 – Senate). The bill was adopted by the Senate, with certain modifications, on 17 May 1962, and forwarded to the National Assembly (N°175 – National Assembly). However, following a change of legislature, the vote on the bill was delayed.97 The Minister of Labour at that time, Mr Grandval, took advantage of this delay to make some relaxations to the proposed directives, and had written to the Regional Directors of Social Security. He did not consider it correct to maintain reimbursement for the polio vaccination only under the guise of epidemic threats, or of direct danger of contamination to the insured. In other words, reimbursement for polio vaccination should be generalised. He also no longer wanted reimbursement to be conditioned by vaccination in Public Vaccine Centres. There was no longer any question of refusing reimbursement for vaccinations by private practitioners.98 This meant that before 1962, primary health insurance funds were not required to reimburse polio vaccination if it was done outside of PVCs. In any case, in May 1963, the sale of the live polio vaccine was not yet authorised in France,99 and the Lépine vaccine, in whose approval process Sohier was involved, had been on the point of losing its licence, which finally did not happen.100 Throughout 1963, 738 cases of paralytic polio were still recorded, with a morbidity rate of 1.62 per 100 000 and seventy deaths. Vaccination was still being carried out with the French inactivated vaccine, following the same procedure, but a booster injection was given with the combined polio, diphtheria and tetanus vaccine developed by Mérieux.
Following the change of legislature, a new bill was introduced on 1 October 1963.101 However, it would be necessary to wait for the law of 1 July 1964 for polio vaccination to be made compulsory.102 At that time, this compulsory vaccination only concerned the Lépine vaccine, but in January 1965 that changed. The polio vaccination to which all people under the age of 30 were subject had to include either a series of three injections and a booster injection, or three oral doses (of trivalent vaccine or of monovalent vaccine but in this case each type of viruses I, II and III had to be administered at least once) and two doses of trivalent vaccine. However, at that time, in January 1965, only type I and type II vaccines had approval, and only type I was authorised for production. Using virus I alone, or viruses I and II seemed to raise many drawbacks: it was unsatisfactory from the point of view of prophylaxis because type III was circulating in France, and interfered with the catching up of young people under 30 years old. This vaccination, as envisioned, ultimately risked undermining the correct application of the law and hampering the rapid spread of immunisation while at the same time incurring significant costs.103 Nevertheless, French polio morbidity rates decreased considerably (0.59 per 100 000 in 1965, 0.427 per 100 000 in 1966 and 0.228 per 100 000 in 1967) after vaccination was made compulsory (Table 2).104
Meanwhile, the Institut Mérieux had written to the RIT laboratory, to the Istituto Sieroterapico e Vaccinogeno Toscano and to Behringwerke, to find out the prices charged in the public market for the sale of the oral trivalent vaccine.105 Such a manoeuvre was clearly aimed at offering a fair price in relation to the monetary value given to the vaccine in other European countries, when the OPV would be authorised for sale in France. In February 1965, a further preparatory action by Charles Mérieux was to request the intervention of Pierre Lépine with the Superior Council of Hygiene and the Academy of Medicine in favour of the Mérieux vaccines, in view of the imminent decree of compulsory polio vaccination.106 One month later, decree N°65–213 of 19 March 1965 was passed to implement the law of 1 July 1964 on compulsory polio vaccination.107 In April 1965, formalities still had to be settled for the approval of the oral trivalent vaccine. The aim of this approach was to promote its systematic use before the end of the year. The administrative and technical difficulties already encountered by physicians for the application of the oral vaccine seemed to have seriously delayed the procedure.108
A new procedure and the attempted vaccination campaign in Lyon
Following a meeting held in the office of the Director General of Public Health in September 1965, it was admitted that individual vaccination with the Sabin vaccine could not be ruled out. On the one hand, the Sabin vaccine was going to be on sale in pharmacies before the end of the year; on the other hand, families should be free to entrust this vaccination to their attending physician. However, it seemed problematic to ask practitioners to summon patients three times to their office to control the absorption of the oral vaccine.
To overcome this constraint, the Institut Mérieux had proposed a procedure for the application of the Sabin vaccine in daily practice.109 The physician would write a prescription for the vaccine, while certifying that there was no contraindication. The pharmacist would ensure the necessary cold storage of the vaccine and could request that the administration of the three basic doses and the boosters take place in his or her presence. Thus, the physician’s prescription, supplemented by the pharmacist’s certificate, could constitute a valid certificate for vaccination by the Sabin method.110 For children under 1 year old, it seemed logical to stick with the injected vaccine, which allowed the necessary vaccine associations to facilitate the schedule. For older children and adults, group vaccination sessions could be planned in schools, barracks, factories etc.111 This was a unique opportunity to involve the physician and the pharmacist in this operation.
In order to prepare for this procedure, the Institut Mérieux wrote to the president of the College of Physicians, to the president of the College of Pharmacists, and to various figures from the world of health in France.112 It also asked the president of the College of Pharmacists to contact the president of the College of Physicians directly to reach an agreement. Charles Mérieux was convinced that the dispensary pharmacist could not only help to popularise the vaccine, but also ensure that it was applied more effectively than in PVCs. In addition, he thought the family physician would prefer to issue a prescription after a medical examination rather than ‘churn out’ 70–80 children per hour as implied in public immunisation circulars.113 Charles Mérieux had, in fact, led a major communication campaign between different actors. However, as he said himself: ‘since 1961, the development of the Sabin has given us so much concern that I am concerned about its distribution’.114 It was essential to distinguish clearly, on the one hand, the inactivated vaccine (Lépine), preferably in its tetravalent form (D.T. Coq-Polio) officially appearing in the vaccination schedule. Several figures in the health world were of the opinion that inactivated vaccines should remain compulsory in very young children, so as to facilitate combinations and not to neglect toxoids (namely diphtheria and tetanus), as well as pertussis. On the other hand, the oral vaccine would initially be recommended for boosters and group vaccinations.115
However, the physician–pharmacist combination did not seem to be as obvious as Charles Mérieux had hoped, given the connection with broader issues of medical and pharmaceutical expertise and authority, which had been so important from the start of vaccination practice.116 Indeed, he had received an objection from the College of Physicians, saying that: ‘a vaccination certificate constitutes a medical act regardless of the method of said vaccination’. Under these conditions, it seemed impossible for the physicians to respond favourably to the proposal made by the director of the Lyon institute.117 Mérieux responded by insisting on the need for medico-pharmaceutical twinning for the Sabin vaccine. The main argument raised was that, at a time when massive polio vaccination catchups were being organised in factories, schools, barracks etc. and whose physicians were excluded, it was necessary to keep the possibility of family vaccination (possible thanks to the oral vaccine).118 Despite the refusal of the College of Physicians, the Lyon institute did not want to drop the case. Charles Mérieux had addressed a letter to Dr Raoul Kourilsky, professor of clinical medicine at Saint-Antoine hospital in Paris, and an influential person in health policy in France.119 Mérieux reiterated his support for the waste of time the administration of the oral vaccine could represent for the physician: ‘it is difficult for the family physician to count fees and waste his time attending the ‘tasting’ of the vaccine’; but also the interest of involving the pharmacist: ‘the pharmacist being quite happy to ‘sublimate’ his diploma by having a vaccine drunk in front of him, especially since it must be kept exclusively at +4° for a maximum of 2 months!’. He therefore contacted Kourilsky for his support, and for the latter to convey the message that, in a way, the physician was free to make a tacit agreement with the pharmacist. In addition, he added that the OPV could be bought freely in pharmacies without a medical prescription. More critically, he had no qualms in saying that some physicians would most certainly issue certificates of convenience, as was already the case with other vaccinations (tuberculosis and tetanus).120
Unfortunately for Mérieux, Kourilsky did not share his opinion on the matter.121 One day before Mérieux’s letter, on 21 December 1965, in his speech to the Academy of Medicine, Kourilsky clearly laid out his position on the subject, and the six key points of the guidelines for use of polio vaccines in France from then on. Among other things, this included the administration of oral vaccine to all age groups, its systematic use both for the primary vaccination and for the booster, and to complete treatment begun with Lépine’s inactivated vaccine. Kourilsky ended his presentation by recalling the obligation of the physicians to inform the health authority of any incident or case of poliomyelitis in vaccinated patients, whether it was a vaccinator, a general practitioner or a public health doctor, as set out in the circulars DGS/HP/508/3 of 1 June 1960 and DGS/HP/1667/3 of 8 November 1963.122 Regardless of this new setback, at the beginning of December 1965, a meeting preliminary to the launch of the Sabin polio vaccine had been organised at the Institut Mérieux. The main objective of this meeting was to reflect on the submission to the Ministry of Public Health of a proposal for a national vaccination plan.123
In January 1966, Dr Violet, director of the Lyon Hygiene Office, announced in a press conference the start in the Rhône department of the polio vaccination campaign with the Sabin vaccine, in factories, in schools and in vaccination centres. This presentation took place in the presence of professor Sohier and the presidents of the College and Syndicate of Physicians.124 For the previous 2 years, the Sabin vaccine had made it possible to carry out emergency vaccinations in many French departments. The Institut Mérieux had graciously made the OPV available to the Departmental Directorates of Health. The generalisation of the attenuated vaccine was to be gradual in France. To achieve this, the Lyon institute sent a letter to various health figures to organise an informative meeting. The ultimate goal seemed to be the organisation of a vaccination campaign in each region of France.125 For 5 years, the Institut Mérieux alone had borne all the scientific and material risks of the technical and industrial development of the Sabin vaccine.126 On 21 January 1966, the city of Lyon officially started the polio vaccination campaign; however, it did not have the expected success.127 Among other things, rumours circulated about a negative interaction with the BCG vaccine (the director of the BCG centre in Lyon was talking about closing the centre for a few months).
Charles Mérieux had written to Dr Auregan, the Regional Inspector and Technical Advisor at the Department of Pedagogy, School Education and Guidance of the National Ministry of Education, concerning the compulsory application in France of the Sabin polio vaccine. As it was a collective vaccination, this was – for Mérieux – a problem of national education. He therefore asked Dr Auregan to take a positive interest in this vaccination and facilitate its execution.128 As Technical Advisor, Auregan told him that he was not empowered to take decisions, but could usefully contact the relevant departments of the Ministry of National Education so that a position could be defined, if necessary in principle, particularly concerning the use of school premises for this vaccination.129 However, in the meantime, Mérieux had not given up on the idea of setting up the medico-pharmaceutical twinning for the administration of the Sabin vaccine.130 Finally, at the end of January 1966, an agreement had been reached during the meeting of the colleges of physicians and pharmacists regarding oral polio vaccination. The members of the two colleges had agreed that the vaccination should be done under the responsibility of the physician and on his prescription; the pharmacist would issue a certificate supplemented by the physician’s medical certificate.131 As the polio vaccination campaign was not having the expected success and was taking time to develop, this presented an opportunity for the medical practitioner to play a key role in the compulsory oral polio vaccination.
At least from 21 February 1966 (circular N°120), polio vaccination could also be carried out orally using the Sabin vaccine, in accordance with the provisions of Article 2 of the decree of 19 March 1965 (Official Journal 23 March 1965).132 France had monovalent type I, II and III vaccines, and a trivalent vaccine had received legal authorisation. Instructions drawn up after consultation with the National Academy of Medicine and the Superior Council of Hygiene set out the technical rules applicable to oral polio vaccination. They should be distributed to all physicians responsible for compulsory vaccinations. Despite the gradual decrease in polio morbidity in France since the development of vaccination, 290 cases had been declared for 1965, while several neighbouring countries where mass vaccination of the population had been carried out now showed only very rare cases of polio, such as were the cases of Denmark, Sweden and Switzerland (Table 2). The circular therefore insisted on the need to develop at the departmental level a program allowing rapid polio vaccination of the greatest number of people.133 Moreover, in March 1966, the Health and Social Action Committee had coordinated the sending of circulars and posters to inform the medical and pharmaceutical profession about the polio vaccination.134 After the unsuccessful experience of the mass campaign in Lyon, Charles Mérieux had written to Minister Aujoulat that it was vital that an oral polio vaccination campaign be launched nationally. If one took into account that three administrations were required, 2 months apart, the vaccination should be started in January or February 1967.135
Conclusion
The road to polio vaccination in France was long, and in several stages. The trigger was undeniably the work of Pierre Lépine; more specifically, his foreign research stays in the US (laboratory of Jonas Salk) and in Canada (the Connaught Laboratory and the Institute of Microbiology and Hygiene of the University of Montreal) in order to improve the work on the culture of poliovirus, and the selection of different safer strains for producing a vaccine. These foreign scientific contacts, together with his previous training in the Institut Pasteur, were decisive for the development of the double inactivation procedure (with formaldehyde and propiolactone), leading to a vaccine safer than, and just as effective as, the Salk vaccine. The French style of polio vaccine innovation was close to the more cautious British and Swedish pattern, and different from the urgency of the development of the US vaccine. Paradoxically, the Lépine vaccine never had the success of Salk’s vaccine, despite its greater safety, without losing efficacy. Perhaps this peculiarity can be explained by the lack of support of the French institutions at the beginning. In the United States, polio was certainly a major public health issue during the second half of the twentieth century.136 Everything was done to fight against the disease, and the National Foundation for Infantile Paralysis was a phenomenal support. In France, Lépine was isolated and one of the few to work on the subject. He was forced to threaten his institutions – to relocate the production of Lépine vaccine to Canada, as well as to obtain the support of Anthony Payne from the WHO and the designation of his laboratory as part of the network of WHO poliomyelitis regional centres – to really draw attention to vaccination in France. Subsequently, the French health authorities realised the urgency of dealing with polio and the damage it could cause.
However, it would be too easy to attribute the fault only to the French health authorities. At this time, the Institut Pasteur still had an important influence to instil the research priorities. In addition, as Gaudillière has already noted, the engagement of Lépine for the development of a polio vaccine was late. Until 1953, Lépine worked on the polio immunity, but all his public interventions aimed to explain that faced to an epidemiological situation extremely complex with polio, no vaccine strategy could be effective in a foreseeable future.137 So it would be fairer to say that the moment was ripe between the Lépine’s work, the international support and some awareness of the disease. However, the industrial scale production of the Institut Pasteur was insufficient to ensure the protection of the population. Here, the intervention of the Institut Mérieux, a private pharmaceutical laboratory, and, particularly, the actions and manoeuvres of Charles Mérieux, as well as his national and foreign contacts, were decisive.
Charles Mérieux and the Institut Mérieux were sensitise at the question of polio during the First International Congress on Biological Standardization, organised by the Lyon institute in 1956. There was an ambivalent relation between the two institutes. They both worked together to mobilise against the fight of the disease, but there were some tensions concerning the Lépine vaccine production. This ambivalent relationship marks the history of Mérieux family. Marcel Mérieux – the father of Charles – was formed at the Institut Pasteur by Émile Roux (1853–1933). After that, he founded his own institute in Lyon. Marcel and Charles – who also took teachings in the Paris institute – have always claimed to be in the Pasteurian tradition. All his career, Charles Mérieux tried to associate the two institutes in common actions and projects, but with varying degree of success, which may be explained by the interaction of two different logics and interests. As seen in this study, the tensions appeared concerning the distribution of Lépine vaccine production, which clearly shows the predominance of the private interests of the French pharmaceutical company, Mérieux, over the Institut Pasteur in managing the vaccination of the population. Furthermore, to overcome this issue, the Institut Mérieux sought to be the first producer of OPV in France, but without giving up the Lépine vaccine and ensuring that the Institut Pasteur would not be a rival as it was with the French vaccine. Maybe in a stranger way, the Lyon institute did not hesitate to defend Lépine and his vaccine – with regard to American vaccines – in the SV40 virus controversy. While as the only producer of OPV in France, it could have been advantageous to defend this product instead. However, it is true that at this moment, the OPV could not be marketed, the Lépine vaccine therefore being the only one vaccine on the market. Where Charles Mérieux was clever was when he proposed to associate the polio vaccination with the D.T. vaccinations already in place. This manoeuvre facilitated the compulsory and booster vaccination of diphtheria and tetanus (in a context where the vaccine coverage for these two diseases was insufficient), and incorporated the Lépine polio vaccine in the valences. Subsequently, the use of D.T. Polio or D.T. Coq-Polio became the norm, a combined vaccine developed, produced and marketed by the Institut Mérieux. In this way, it eliminated a large part of the competition from the Institut Pasteur in the market of the French vaccine.
If the two institutes and the two main protagonists worked together, they each had different influences, at different times with the aim to raise awareness of the fight against the disease. The Institut Pasteur and Lépine paved the way in the 1950s. The Institut Mérieux and Charles Mérieux took over in the 1960s. Charles Mérieux activated his national and international networks – not only medical/scientific, but also political and educational – in order to mobilise against the disease, and had significant commercial revenue. Different actions and manoeuvres were implemented for this purpose, mainly: reimbursement of polio vaccination by Social Security, compulsory polio vaccination and physician–pharmacist association for the administration of OPV. In fact, all these strategies were adopted, even if the last one did not last long. The Lyon institute and Charles Mérieux were important to trigger these trajectories. However, to what extent the lobbying was instrumental in the implementation of these measures remains a question without a clear answer. It is certain that the study of the archives of certain organisations cited and involved in polio vaccination could provide additional answers. Anyway, it is obvious that other protagonists – not evoked in the text – had a determining role, especially in the medical and political spheres.
What sort of information does this study bring to the understanding and consideration of polio by health authorities in France in the 1950s and 1960s? As already mentioned, polio was considered less pressing than other diseases such as diphtheria, tuberculosis or complications from eruptive fevers.138 These diseases were studied by the different departments of the Institut Pasteur. Thereby, it was the Paris institute which instilled the way forward. And this is confirmed with the work of Lépine on polio thereafter. If polio vaccination started relatively quickly in 1956 after the development of the first effectives polio vaccines, the steps for an active vaccination were delayed. The law for compulsory polio vaccination was enacted on 1 July 1964 and implemented on 19 March 1965. Ten years separate the development of the Lépine vaccine and the compulsory polio vaccination. An additional proof concerning the perception of the disease by the French health authorities.139 Especially since the disease was easily controlled by mass vaccination in certain neighbouring European countries, and that the incidence in France did not decrease so significantly (again, compared to other European countries). In 1957, the morbidity rate of polio was 9.32 per 100 000 inhabitants.140 Six years later, in 1963, 738 cases of paralytic polio were still recorded, with a morbidity rate of 1.62 per 100 000 and seventy deaths. At this moment in Europe, only Spain had a worse result than France.141 The French health authorities initially tried to solve the problem by setting up PVCs, but they never had the performance that the family physician had to administer vaccines to be more consistent with the French health care tradition. Therefore, it was the establishment of compulsory polio vaccination that was decisive. This measure was supplemented by the use of the combined D.T. (Coq-)Polio vaccine in the vaccination schedule as in the Netherlands, but also by the use of the OPV to make up for the delay in vaccination rate, in particular through the organisation of vaccination campaigns in France.
Ultimately, the polio vaccines (Lépine vaccine and OPV) were essential for the Pasteur and Mérieux institutes in order to mobilise against the disease. Mobilisation against polio in France, as it was led by the two institutes, only materialised through vaccination. Poliomyelitis was perceived and considered only in connection with its best means of prevention, namely vaccines. This led to the construction of a situation where only the success and generalisation of polio vaccination in France mattered. The main obstacle in the end was the failure to prevent it. In the perception of the disease, its impact and its severity, there was therefore an association, even confusion, between polio on the one hand, and collective biotechnological protection on the other. The historical processes by which this disease has been confused with a biotechnology of collective protection, on a national scale but perhaps also on a global scale, still remain strangely unexplored, and would deserve a renewed interest to analyse the problems and the stakes that they raise.
Acknowledgement
This paper was produced within the context of the research projects ‘Grant Programmes for Research Visits and the Role of Public and Private Laboratories in The Fight Against Infectious Diseases in Europe (1907–85)’.
Funding
This research was funded by the State Agency of Research (AEI), the Ministry of Science and Innovation, Spain (MICIN)–FEDER Funds (ref. PID2019-108813GB-I00) and by the Board of Community of Castilla-La Mancha (Spain)–FEDER Funds (ref. SPBY/17/180501/000382).
Competing interests
The authors declare none.





