Research Article
A note on k-connexes
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. 129-143
-
- Article
- Export citation
Some relations between certain methods of summation of infinite series
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. 144-164
-
- Article
- Export citation
On Hausdorff's methods of summability
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. 166-192
-
- Article
- Export citation
On Lorentz invariance in the quantum theory
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. 193-200
-
- Article
- Export citation
On Lorentz invariance in the quantum theory. II
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. 201-209
-
- Article
- Export citation
Two-centre integrals occurring in the theory of molecular structure
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. 210-223
-
- Article
- Export citation
On the determination of intermolecular energies by inductive analysis
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. 224-230
-
- Article
- Export citation
Research Notes
Note on the degeneration of algebraic varieties
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. 231-233
-
- Article
- Export citation
A remark on a property of a special pencil of quadrics
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. 234-235
-
- Article
- Export citation
The thermodynamic derivation of Fowler's adsorption isotherm
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. 236-239
-
- Article
- Export citation
Front matter
PSP volume 38 issue 2 Cover and Front matter
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. f1-f2
-
- Article
-
- You have access
- Export citation
Back matter
PSP volume 38 issue 2 Cover and Back matter
-
- Published online by Cambridge University Press:
- 24 October 2008, pp. b1-b2
-
- Article
-
- You have access
- Export citation

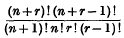
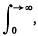 or possibly
or possibly 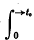 if
if