Published online by Cambridge University Press: 24 October 2008
It is well known that the rotational specific heat of a diatomic gas is given by
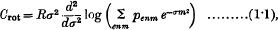
where R is the gas constant, σ = h2/8π2AKT, h is Planck's constant, T is the absolute temperature, K is Boltzmann's constant, and A is the moment of inertia of the molecule.
* Fowler, R. H., Statistical Mechanics, p. 34.Google Scholar
† Bulletin of the National Research Council, 1926.
* The usual notation is ![]() , but I have used a and b here to avoid confusion with
, but I have used a and b here to avoid confusion with ![]() . The formula was thought at one time to have a theoretical basis but is now known to be really empirical.
. The formula was thought at one time to have a theoretical basis but is now known to be really empirical.
* Mulliken, , Physical Review, Vol. 28, p. 1221 (1926).Google Scholar
† Ibid. Vol. 30, p. 789 (1927).
* Proc. Camb. Phil. Soc. Vol. 24, p. 280 (1928).CrossRefGoogle Scholar
† Fowler, , Statistical Mechanics, p. 53.Google Scholar
‡ Whittaker, and Watson, , Modern Analysis, 3rd ed. p. 119.Google Scholar
* Whittaker, and Watson, , Modern Analysis, 3rd ed. p. 126.Google Scholar
* Mulliken, , Reviews of Modern Physics, Vol. 1, p. 105 (1930).Google Scholar
† Journal of American Chemical Society, Vol. 51, pp. 2300, 3194 (1929).CrossRefGoogle Scholar
* Van Vleck, , Physical Review, Vol. 28, p. 1016 (1926).CrossRefGoogle Scholar
† Journal American Chem. Soc. Vol. 51, p. 2317 (1929).Google Scholar
* Journal American Chem. Soc. Vol. 48, p. 1520 (1926).CrossRefGoogle Scholar
† Ibid. Vol. 45, p. 2277 (1923).
‡ Ibid. Vol. 51, pp. 2300, 3194 (1929).
* Mulholland, loc. cit.