Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Penney, W G
1935.
The quantum theory: Part 3. The quantum theory of valency.
Reports on Progress in Physics,
Vol. 2,
Issue. 1,
p.
51.
Van Vleck, J. H.
and
Sherman, Albert
1935.
The Quantum Theory of Valence.
Reviews of Modern Physics,
Vol. 7,
Issue. 3,
p.
167.
Hirschfelder, J.
Eyring, H.
and
Rosen, N.
1936.
I. Calculation of Energy of H3 Molecule.
The Journal of Chemical Physics,
Vol. 4,
Issue. 2,
p.
121.
Hirschfelder, J.
Eyring, H.
and
Rosen, N.
1936.
II. Calculation of Energy of H3+ Ion.
The Journal of Chemical Physics,
Vol. 4,
Issue. 2,
p.
130.
Stevenson, David
and
Hirschfelder, Joseph
1937.
The Structure of H3, H3+, and of H3—. IV.
The Journal of Chemical Physics,
Vol. 5,
Issue. 12,
p.
933.
Coulson, C. A.
1937.
The evaluation of certain integrals occurring in studies of molecular structure.
Mathematical Proceedings of the Cambridge Philosophical Society,
Vol. 33,
Issue. 1,
p.
104.
Hirschfelder, Joseph O.
1938.
The Energy of the Triatomic Hydrogen Molecule and Ion, V.
The Journal of Chemical Physics,
Vol. 6,
Issue. 12,
p.
795.
Penney, W G
1939.
The theory of molecular structure.
Reports on Progress in Physics,
Vol. 6,
Issue. 1,
p.
212.
Magee, John L.
1940.
Directed Valence in Chemical Reactions.
The Journal of Chemical Physics,
Vol. 8,
Issue. 9,
p.
677.
Mulligan, Joseph F.
1951.
LCAO Self-Consistent Field Calculation of the Ground State of Carbon Dioxide.
The Journal of Chemical Physics,
Vol. 19,
Issue. 3,
p.
347.
Barker, Roland S.
and
Eyring, Henry
1954.
Use of Nuclear Attraction Integral Approximations in Molecular Quantum Mechanics.
The Journal of Chemical Physics,
Vol. 22,
Issue. 7,
p.
1182.
Barker, Roland S.
Giddings, J. Calvin
and
Eyring, Henry
1955.
Energy Calculations for the Linear H3+ Ion System*.
The Journal of Chemical Physics,
Vol. 23,
Issue. 2,
p.
344.
Ellison, Frank O.
1955.
Molecular Calculations. II. Methods of Approximation of Molecular Integrals.
The Journal of Chemical Physics,
Vol. 23,
Issue. 12,
p.
2358.
Polanyi, J. C.
1959.
Energy Distribution Among Reagents and Products of Atomic Reactions.
The Journal of Chemical Physics,
Vol. 31,
Issue. 5,
p.
1338.
Sutton, L. E.
1961.
Chemische Bindung und Molekülstruktur.
Vol. 3,
Issue. ,
p.
28.
Conroy, Harold
1964.
Potential Energy Surfaces for the H3+ Molecule-Ion.
The Journal of Chemical Physics,
Vol. 40,
Issue. 2,
p.
603.
Conroy, Harold
1964.
Molecular Schrödinger Equation. IV. Results for One- and Two-Electron Systems.
The Journal of Chemical Physics,
Vol. 41,
Issue. 5,
p.
1341.
Pearson, A. G.
Poshusta, R. D.
and
Browne, J. C.
1966.
Some Potential-Energy Surfaces on H3+ Computed with Generalized Gaussian Orbitals.
The Journal of Chemical Physics,
Vol. 44,
Issue. 5,
p.
1815.
Joshi, Bhairav D.
1966.
Study of the H3+ Molecule Using Self-Consistent-Field One-Center Expansion Approximation.
The Journal of Chemical Physics,
Vol. 44,
Issue. 9,
p.
3627.
Kadura, P.
1967.
Moddellrechnungen zur symmetrischen Vierzentrenbindung. I.
Theoretica Chimica Acta,
Vol. 7,
Issue. 5,
p.
433.
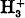
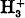 in its ground state and excited levels, and the relative importance of the perturbation and variational methods has been considered in some detail, as well as the effect of certain integrals which, in discussions of molecular structure, have often been neglected. It appears that the ion
in its ground state and excited levels, and the relative importance of the perturbation and variational methods has been considered in some detail, as well as the effect of certain integrals which, in discussions of molecular structure, have often been neglected. It appears that the ion 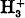 should exist in stable equilateral form with a nuclear distance about 0·85 Å., and that all excited levels are unstable.
should exist in stable equilateral form with a nuclear distance about 0·85 Å., and that all excited levels are unstable.

