Recently, Yahr (Reference Yahr2015) studied British populations of Calicium corynellum (Ach.) Ach. to test whether these were distinct from C. viride Pers. As C. corynellum had the highest conservation priority in Britain, and was apparently declining rather dramatically, it was important to clarify its taxonomic status. Yahr (Reference Yahr2015) used both genetic (ITS rDNA) and morphological data from two British Calicium aff. corynellum populations and could not find differences with C. viride, suggesting that the British material represented saxicolous populations of the otherwise epiphytic or lignicolous C. viride. Although this study focused on British material, it introduced serious doubts about the identity and relationships of these two species in other parts of the distribution area of C. corynellum. Interestingly, the British material that Yahr (Reference Yahr2015) investigated was morphologically very similar to C. viride, but the latter was described as differing rather substantially from C. corynellum in other parts of its distribution area. Thus, C. corynellum differs from C. viride in its short-stalked, greyish white pruinose ascomata (C. viride has long stalks and a brown pruina), distinctly narrower spores compared with C. viride, and the leprose thallus (Fig. 1) which is granular to verrucose in C. viride (Tibell Reference Tibell, Ahti, Jørgensen, Kristinsson, Moberg, Søchting and Thor1999).

Fig. 1. Calicium corynellum habitus (M. Prieto C4 (ARAN-Fungi 8454)). Scale = 1 mm. In colour online.
Calicium viride is a common and widely distributed species in temperate areas of the Northern Hemisphere and southern South America (Tibell Reference Tibell, Ahti, Jørgensen, Kristinsson, Moberg, Søchting and Thor1999; GBIF 2019). Calicium corynellum is, however, not as well understood, with scattered occurrences in Europe and North America (Sarrión et al. Reference Sarrión, Aragón and Burgaz1999; Tibell Reference Tibell, Ahti, Jørgensen, Kristinsson, Moberg, Søchting and Thor1999; Pérez-Ortega Reference Pérez-Ortega2007; GBIF 2019). Additionally, C. corynellum is currently being evaluated for the Red List of lichens of Spain and Portugal (Atienza et al. Reference Atienza, Fos, Burgaz, de Silanes ME, Millanes, Olariaga, Paz-Bermúdez, Pérez-Vargas, Prieto and Pérez-Ortega2019), which makes it essential to assess the taxonomic status of the species.
As C. corynellum is such a poorly known species and the results obtained by Yahr et al. (Reference Yahr2015) may be interpreted to suggest that C. corynellum could be better treated as a synonym of C. viride, the status of C. corynellum should be studied further. Our aim here is to study the delimitation of these two taxa based on a larger sample, covering the whole known distribution range of C. corynellum. For that purpose, we have conducted a phylogenetic analysis based on sequences of the ITS region of the nuclear rDNA.
We sampled our own recently collected material, several herbarium specimens and a selection of samples from GenBank (Table 1). Calicium salicinum Pers. was included as outgroup based on Prieto & Wedin (Reference Prieto and Wedin2017). DNA was extracted and amplified (nrITS region) following Prieto & Wedin (Reference Prieto and Wedin2017). Sequences were assembled and edited using Sequencher v. 4.10.1 (Genes Codes Corporation, Ann Arbor, MI, USA) and deposited in GenBank (Table 1). Subsequently, sequences were aligned manually using MacClade 4.01 (Maddison & Maddison Reference Maddison and Maddison2001). No ambiguous regions and introns were found. A phylogenetic analysis was carried out using maximum likelihood-based inference (ML) as implemented in RAxML v. 8.2.10 (Stamatakis Reference Stamatakis2014) run on the CIPRES Science Gateway v. 3.3 (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). The analysis was performed with a GTRGAMMA model for tree inference and GTRCAT model and 1000 replicates for bootstrapping.
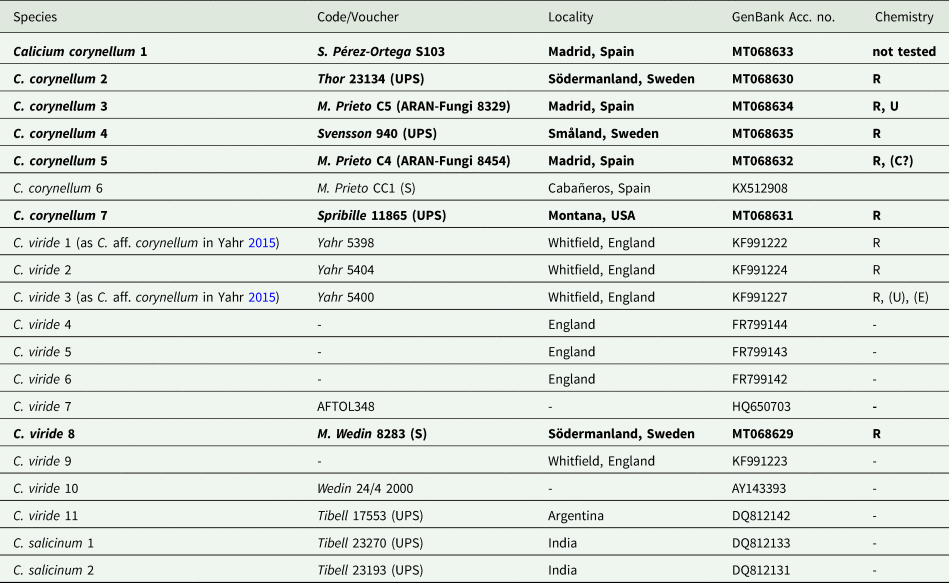
Table 1. Calicium specimens used in this study, with GenBank Accession numbers. Entries for newly obtained sequences and new chemical analyses are in bold. Specimen data are given for newly produced sequences with collection number and location of voucher. Chemistry: R = rhizocarpic acid, U = usnic acid, E = epanorin, C = calycin. Codes enclosed by parentheses indicate trace amounts. Data from specimens not analyzed here come from Yahr (Reference Yahr2015).
Thin-layer chromatography was carried out following standard methods (Orange et al. Reference Orange, James and White2001) and using solvent systems B and C. As data given in the literature (i.e. Tibell Reference Tibell, Ahti, Jørgensen, Kristinsson, Moberg, Søchting and Thor1999) refer to the thallus and not to the ascomata, we sampled thallus parts.
A total of seven sequences were newly generated for this study (Table 1). The data set consisted of 20 taxa and 486 unambiguously aligned sites. The ML tree with bootstrap values is shown in Fig. 2 and demonstrates that samples classified as Calicium corynellum and C. viride based on morphology form two distinct monophyletic groups, which we interpret here as different species. While there is some genetic variation within Calicium viride, C. corynellum has very little (Fig. 2).

Fig. 2. Phylogenetic tree (best maximum likelihood (ML) tree) of Calicium corynellum and Calicium viride resulting from RAxML analysis of nrITS sequences. Bootstrap supports (ML-BS) are shown above branches and supported clades (ML-BS ≥ 70) are marked with thicker black branches. Calicium salicinum is used to root the tree.
Our results suggest that Calicium corynellum is a distinct species which can be distinguished from C. viride by both morphological and genetic characters. Our results also support that the two British populations studied by Yahr (Reference Yahr2015) correspond to specimens of C. viride growing on rocks. However, this does not exclude the possibility that the real C. corynellum might also exist in Britain.
The two species also apparently differ chemically, as C. viride contains rhizocarpic acid and epanorin, and C. corynellum contains rhizocarpic and usnic acids (Tibell Reference Tibell, Ahti, Jørgensen, Kristinsson, Moberg, Søchting and Thor1999; Yahr Reference Yahr2015). This was not evident in the study by Yahr (Reference Yahr2015). Of the samples collected by Yahr (C. aff. corynellum) that grew on rock, only three out of seven chemically tested specimens contained usnic acid. From the five herbarium specimens chemically studied by Yahr (probably representing the real C. corynellum), one did not have usnic acid. The C. corynellum specimens sampled here contained only rhizocarpic acid, except one sample that also contained usnic acid (Table 1). Similar to Yahr, we did not detect epanorin in the C. viride specimen studied. Our results further support that the amounts of usnic acid in C. corynellum and epanorin in C. viride are at least very variable (and that usnic acid may also occur in saxicolous C. viride) and difficult to use to identify these species, just as Yahr (Reference Yahr2015) suggested.
The studied specimens of Calicium corynellum were not lichenicolous and produced well-developed thalli (Fig. 1). The Spanish specimens were all found in a very typical but easily overlooked habitat: overhanging siliceous rocks in shaded and humid situations. The Spanish distribution is mainly along the Central Mountains but, as we have recently found a number of new localities, we believe that the species is probably very overlooked in these areas, as in the rest of the world.
We can conclude that Calicium corynellum is a species distinct from C. viride and thus should be treated separately in future conservation activities and Red List assessments.
Acknowledgements
This project was funded by the Swedish Research Council (VR 2016-03589).
Author ORCIDs
Maria Prieto, 0000-0002-1692-9821; Ibai Olariaga, 0000-0002-0334-7750; Sergio Pérez-Ortega, 0000-0002-0334-7750; Mats Wedin, 0000-0002-8295-5198.






