Introduction
The genus Gibbosporina was described by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016), comprising 13 species distributed through palaeotropical areas from Réunion, Mauritius and Sri Lanka eastwards to islands in the western part of the Pacific. All species look somewhat similar and are rather large foliose lichens dominated by their green major photobiont, but with conspicuous cephalodia. All species are normally abundantly fertile without vegetative dispersal units, except for brittle cyanobiont lobes in several species. The only exception was the sterile G. phyllidiata Elvebakk, which was recently transferred to Pannaria and renamed P. melanesica Elvebakk (Elvebakk & Sipman Reference Elvebakk and Sipman2020). Although resembling tripartite austral Pannaria species, Gibbosporina clearly belongs in a clade with genera such as Physma, Leightoniella and Lepidocollema, as shown in phylograms by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016) and Weerakoon et al. (Reference Weerakoon, Aptroot, Wedin and Ekman2018), and also by Magain & Sérusiaux (Reference Magain and Sérusiaux2014), prior to the description of the new genus. Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016) also showed good molecular support for seven of the new species.
Concerning Sri Lanka, Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016) reported on two studied Gibbosporina collections, both originally deposited as Thwaites 52 in S and determined as G. mascarena Elvebakk et al., although they were found to differ in some characters. The conclusion was that ‘future studies might well discover more than one taxon from this island’. It was therefore exciting when another Thwaites collection of a Gibbosporina from Sri Lanka deposited in BM was discovered. At first glance, it appeared similar to a classic Gibbosporina with small-foliose cephalodia, but it soon proved to be a remarkable, bipartite cyanolichen, with cephalodia-like structures as phyllidia-like ramifications of the thallus. The aim of the present paper is to describe this collection as a new species. The new species is more likely to be confused with other cyanogenera within Pannariaceae than with other species of Gibbosporina. To provide clarification, the species is therefore compared here to related cyanolichen genera from the Palaeotropics based on perispore structure, a diagnostic but previously overlooked character.
Material and Methods
Herbarium material used for this study is housed in BG, BM, G, PC, TNS and TROM. Perispore structures were studied in water mounts and restricted to mature spores liberated from asci, and iodine reactions were tested in microscope sections by adding IKI to mounts pretreated with KOH. Ascospore morphology was studied in detail by drawing detailed sketches of c. 150 ascospores with a focus on perispore structures, and copies of the sketches were deposited with the herbarium specimens. The illustrations presented here aim to depict the variations in shape and size within and between the species studied, and, in addition to the new species, specimens were selected to represent neighbouring genera of the genus Gibbosporina, as well as major clades of genera which have recently been shown not to be monophyletic. Thin-layer chromatography of acetone extracts follows standard procedures (Orange et al. Reference Orange, James and White2001), and nomenclature of ascospore structures follows Nordin (Reference Nordin1997).
Additional collections examined
Gibbosporina bifrons Elvebakk et al. Malaysia: Sarawak: Gunong Mulu National Park, 4th Division, Baram District, Long Pala, limestone hill, c. 2 km E of Base Camp, S side of Sungei Melinau Paku, 70‒300 m alt., 1978, G. Argent, B. Coppins 5440, C. Jermy & P. Chai (BG L-32437).
Gibbosporina boninensis (Kurok.) Elvebakk & P. M. Jørg. Japan: Bonin Islands: US North Pacific exp. under command of Ringold and Rodgers 1893‒96, C. Wright s. n. (PC0012753).
Leightoniella zeylanensis (Leight.) Henssen. Sri Lanka: G. H. K. Thwaites 1876 (G 00292260).—Japan: Bonin Islands: Chichijima Island, Takeda pastureland, 1930, F. Fujikawa s. n. (TNS).
Lepidocollema brisbanense (C. Knight) P. M. Jørg. New Caledonia: S part, Village de Prony, near the beach N of the village, 22°19′09″S, 166°49′37″E, on large trunk of dying mango tree, 3 m, 2005, A. Elvebakk 05:559 (TROM).
Lepidocollema marianum (Fr.) P. M. Jørg. New Caledonia: SE part, Touaourou, near the church, 22°11′10″S, 166°58′30″E, on Araucaria columnaris, 10 m, 2005, A. Elvebakk 05:618 (TROM).
Lepidocollema polyphyllinum (P. M. Jørg.) P. M. Jørg. Solomon Islands: Guadalcanal Island: central part, Mt Popomansiu, on ridge SE of Sutakiki River, montane rainforest, 5800–6600 ft, 1965, D. Jackson Hill 9485 (BM000731913).
Lepidocollema stylophorum (Vain.) P. M. Jørg. Réunion: W of St Philippe, below Mare Longue Nature Reserve, 1.5 km up along the road towards the Reserve, 21°21′36″S, 55°44′28″E, 90 m, on trunks along the road, 2011, A Elvebakk 11:093 (TROM).
Pannaria complanata P. M. Jørg. India: Nilgiri Mts, Ootacamund, Dodabetta Peak, in eucalypt forest, c. 3000 m, 1972, K. P. Singh 72126 (BG—isotype).
Pannaria lurida (Mont.) Nyl. USA: Hawaii (‘Sandwich Isl.’): without further data, Gaudichaud, Voy. ‘Bonite’ (BM—lectotype!); Kauai, mountain forest at Waimea Canyon, 21°57′60″N, 159°39′45″W, 900 m, epiphytic, 1988, A. Elvebakk 88:016 (TROM).—Sri Lanka: ‘Ceylon, Central Highlands’, G. H. K. Thwaites, undated, as C.L.9 (BM013392391b).
Physma cf. boryanum (Pers.) A. Massal. Réunion: W of St Philippe, below Mare Longue Nature Reserve, 2.5 km up along the road towards the Reserve, 21°20′30″S, 55°44′27″E, 120 m, on trunks along the road, 2011, A. Elvebakk 11:096, 11:098 (TROM, both previously determined as P. byrsaeum by A. Elvebakk).
Physma byrsaeum (Afzel. ex Ach.) Tuck. Réunion: W of St Philippe, below Mare Longue Nature Reserve, 2.5 km up along the road towards the Reserve, 21°20′30″S, 55°44′27″E, 120 m, on trunks along the road, 2011, A. Elvebakk 11:095, 11:097, 11:099 (TROM, previously determined as P. radians Vain. by A. Elvebakk); Takamaka, 2009, F. Schumm 15272 & J.-P. Frahm (TROM).—Seychelles: Mahé: Morne Seychellois National Park, 2008, F. Schumm 14658 & J.-P. Frahm (TROM, previously determined as P. radians Vain. by A. Elvebakk); Morne Blanc, 2008, F. Schumm 14644 & J.-P. Frahm (TROM).—New Caledonia: 10 km NE of Nouméa, Forêt de Koghi, 1.5 km above the auberge, near Belvedère, 22°10′20″S, 166°30′28″E, 590 m, on the trunk of an unidentified small Araliaceae tree in an open savannah community, 2005, A. Elvebakk 05:694 (TROM, previously determined as Physma sp. by A. Elvebakk).
Physma sp. 1. French Polynesia: Tahiti: Papete, park at Gaugi Museum and Gardens, Papeaivi, 10 m, i 1996, M. P. Jones (BG L-32410; previously as P. byrsaeum (Afzel. ex Ach.) Tuck. by P. M. Jørgensen).
Results
The new species
Gibbosporina cyanea Elvebakk sp. nov.
MycoBank No.: MB 839600
Differs from all other known Gibbosporina species by being a bipartite, phyllidiate cyanolichen, and in addition from G. sphaerospora Elvebakk & Hong by having slightly longer ascospores and nodulose pycnidia.
Type: Sri Lanka, ‘Ceylon, Central Highlands’, G. H. K. Thwaites, undated, as C.L.9 (BM013392391a—holotype).

Fig. 1. Gibbosporina cyanea (holotype). A, the entire holotype. B, details of the phyllidia. Scales = 5 mm. In colour online.
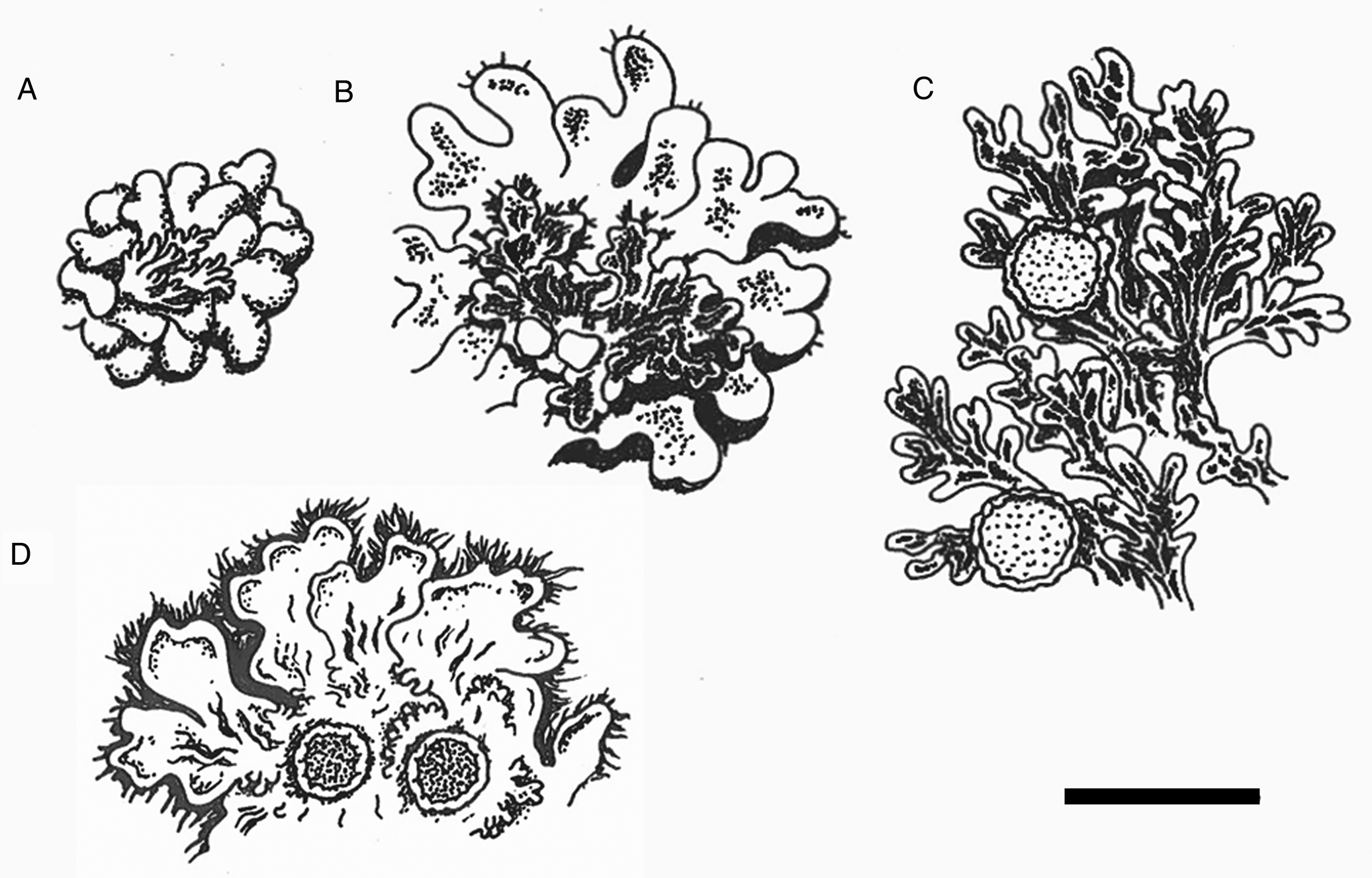
Fig. 2. Sketches of parts of thalli. A, cephalodia on chlorobiont thallus of Gibbosporina boninensis (Wright, PC 0012753). B, cephalodia (with epiphytic chlorobiont squamules) on chlorobiont thallus of G. bifrons (Coppins 5440 et al., BG L-32437). C, cyanothallus of Leightoniella zeylanensis (Fujikawa s. n., TNS). D, cyanothallus of G. cyanea (holotype). Scale = 1 cm.

Fig. 3. Ascospores of Gibbosporina species (B & C based on spore sketches used by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016)). A, G. cyanea. B, G. mascarena. C, G. sphaerospora. Scale = 10 μm.
Thallus a cyanomorph 3–7 cm diam., foliose, corticolous; lobes irregularly and shallowly divided, 150–200 μm thick, c. 2 mm wide, lobes discrete only in peripheral parts, soon becoming coalescent. Upper surface glabrous and matt, with depressions and ridges when dry, probably gelatinous when moist; old herbarium specimens brown, fresh specimens probably greyish. Phyllidia 2–3 mm wide, formed along thallus margins, convex, decumbent and geotropically arranged, 0.2–0.3 mm thick, often with pale rhizomorphs on the lower side, of a bluish grey colour contrasting with the brown main thallus. Upper cortex 10–15 μm thick in the lobules, 30–40 μm in the main thallus, plectenchymatic, lumina up to 5 × 10 μm, globose or irregularly subellipsoid and then arranged perpendicularly to the surface, surface weakly sclerenchymatic; lower cortex absent. Cyanobacterial layer c. 100 μm thick; of Nostoc cells, 3–7 × 2–5 μm, globose to irregular and short-ellipsoid, bluish violet, forming distinct chains, in the main lobes in long chains without glomeruli, elsewhere coiled within 20–80 μm large glomeruli or goniocysts. Medulla of loosely interwoven hyphae, 30–50 μm thick, in lowermost part with brown-pigmented rhizines, fibrous, black, 0.5–1.5 mm long, simple or in bundles. Lower cortex absent.
Apothecia subsessile, laminal, 1–2 mm diam., disc orange-brown; thalline excipulum c. 0.2 mm, indistinctly crenate-striate; epithecium pale brown, 10–20 μm thick, IKI−; hymenium colourless, but strongly IKI+ blue, 80–120 μm thick; hypothecium pale brown, IKI−, 20–30 μm thick; cyanobacterial layer extending below the hypothecium; paraphyses simple to sparingly branched, with slightly swollen apices, septate and much adglutinated; asci clavate, 8-spored, with pronounced, internal, apical, IKI+ blue, amyloid tube structures, 90–110 × 15–20 μm. Proper ascospores colourless, simple, subglobose to short-ellipsoid, 12–15 × 9–12 μm; perispores 15–20 × 13–15 μm, walls mostly 1–1.5 μm thick with scattered small gibbae when mounted in water.
Pycnidia scattered, marginal, brown and nodulose, 0.1 × 0.1 mm, surface finely papillose; spermatia not seen.
Chemistry
No TLC-detectable compounds found.
Etymology
The species is named after its deviating photobiont structure.
Distribution and ecology
Known only from the type collection.
Perispore structures in members of neighbouring Pannariaceae genera
Leightoniella zeylanensis
Proper spores are regularly ellipsoid and 10–13 × 5–7 μm in size, and perispores are 13–18 × 6.5–8 μm. The latter are thin and even and 0.5–1 μm thick in central parts, but swell gradually into c. 2 μm thick, even, nodulose apical extensions, maintaining a roughly ellipsoid outline (Fig. 4A). The spores of the collections from Sri Lanka and Japan have the same pattern, and the species is reported here as new to Japan.
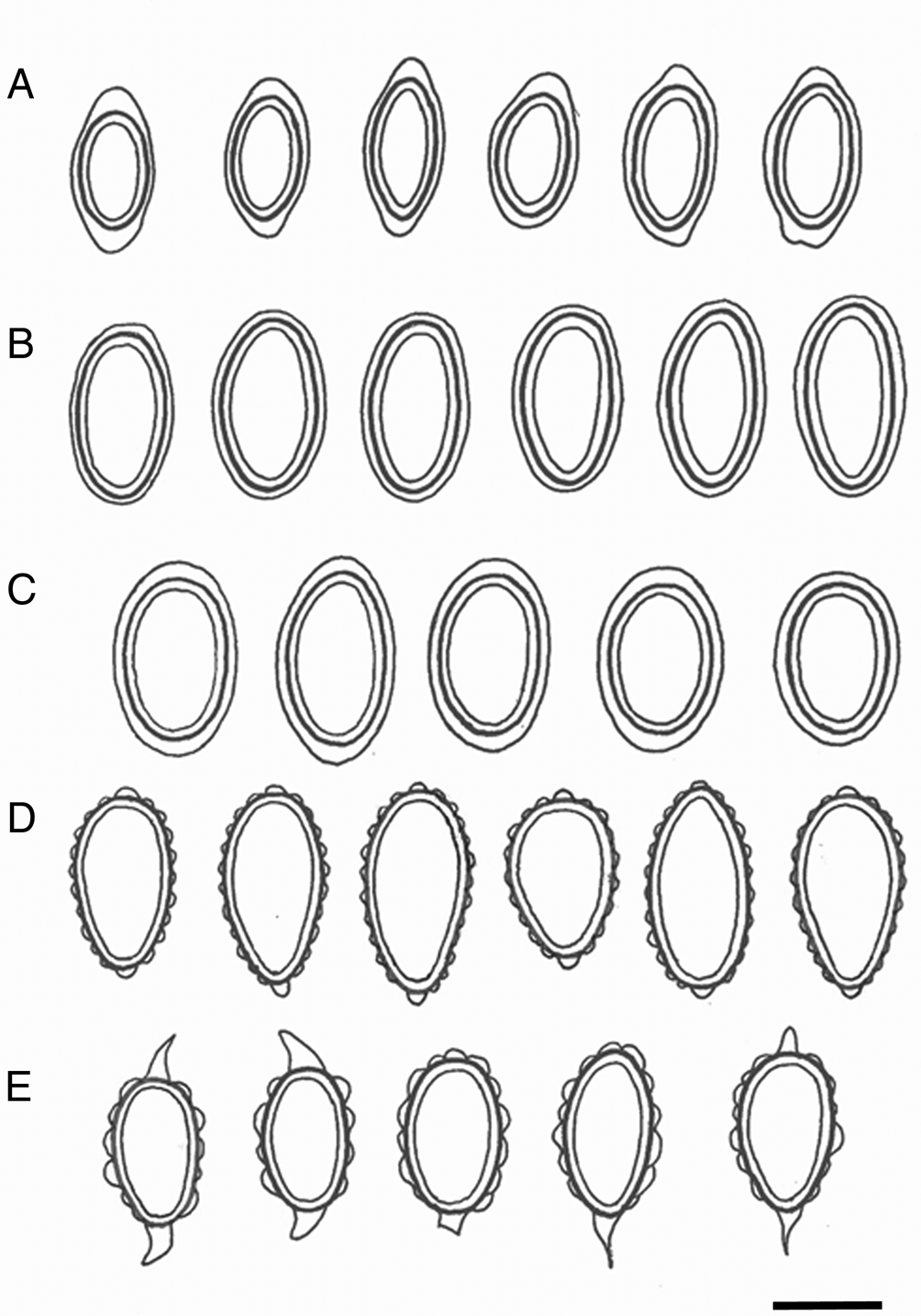
Fig. 4. Ascospores of Leightoniella, Lepidocollema and Pannaria. A, Leightoniella zeylanensis, Sri Lanka (Thwaites 1876, G) and Japan (Fujikawa s. n., TNS). B, Lepidocollema polyphyllinum, Solomon Islands (Jackson Hill 9485, BM). C, L. stylophorum, Réunion (Elvebakk 11:093, TROM). D, Pannaria lurida, Sri Lanka (Thwaites C.L.9, BM013392391b). E, P. complanata, India (Singh 72126, BG). Scale = 10 μm.
Lepidocollema polyphyllinum
Both the proper spores (15–17 × 7–8 μm) and the perispores (16.5–19.5 × 8–9.5 μm) are regularly ellipsoid, and the perispores are smooth and evenly thick, 0.5–1 μm (Fig. 4B).
Lepidocollema stylophorum
Proper spores are regularly ellipsoid (13–17 × 8–10 μm), perispores 16–21 × 10–12.5 μm with gradual transitions to weak apical extensions (Fig. 4C). The ascospores of L. brisbanense are slightly smaller, and with weak apical extensions seen only in some of the spores, but very distinct in immature spores. Lepidocollema marianum has distinct perispore extensions but the spores are shorter and slightly wider, 12–15 × 8.5–10.5 μm.
Pannaria complanata
Proper spores are ellipsoid, 13–16 × 7–8.5 μm, perispores 17–22 × 9–12 μm (Fig. 4E). Mature perispores have acuminate apical extensions, in semi-mature spores they are more diffusely triangular. Perispores have uneven sizes of verrucae, some up to 2.5 μm tall and appearing almost as gibbose structures.
Pannaria lurida
Pannaria lurida is considered a pantropical species, except for ssp. quercicola P. M. Jørg. and ssp. russellii (Tuck.) P. M. Jørg. which occur further northwards, the latter extending into both Canada and easternmost Russia (Jørgensen Reference Jørgensen2000; Ezhkin & Jørgensen Reference Ezhkin and Jørgensen2018). The lectotype of P. lurida ssp. lurida from Hawaii has been studied but is sterile. Other samples from Hawaii have also been studied and correspond in general morphology to the sample from Sri Lanka admixed with the holotype of Gibbosporina cyanea and illustrated in Fig. 4D. Its spores are ellipsoid to ovoid, proper spores 15–19 × 7.5–9 μm, perispores 18–23 × 8.5–10 μm. Perispores have regularly small verrucae and small, nodulose apical extensions. In half of the cases the apical extensions are replaced by normal verrucae. The Sri Lankan specimen deviates from normal P. lurida by having a thick thallus with white margins and with apothecia that are coronate from protruding basal rhizohyphae.
Physma cf. boryanum
One sample from Réunion previously included in the phylogeny by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016) as ‘Physma byrsaeum’ has now been redetermined together with one additional sample from the same locality. The ascospores are illustrated in Fig. 5A. The proper spores are 13–17 × 8–9 μm and regularly ellipsoid. Perispores are 28–42 × 10–12 μm, almost always with two 10–15 μm long, apiculate apical extensions, tapering gradually from an 8–12 μm wide base, equally as broad as the proper spores. In central parts, perispores are only 0.5–2 μm thick and very distinctly gibbose.

Fig. 5. Ascospores of Physma s. lat. species. A, P. cf. boryanum, Réunion (Elvebakk 11:096). B, Physma sp., Tahiti (Jones s. n., BG L-32410). C, P. byrsaeum, Réunion and the Seychelles (based on several cited specimens). D, P. byrsaeum, New Caledonia (Elvebakk 05:694). Scale = 10 μm.
Physma sp. 1 Jones s. n. (Tahiti)
Figure 5B illustrates the spores of this unidentified species. Proper spores are 12–17 × 8–10 μm long and regularly ellipsoid. Perispores are 17–23 × 10.5–12.5 μm long, always with swollen and obtuse apical extensions and 0.5–2 μm thick, gibbose central parts.
Physma byrsaeum
Figure 5C is based on 100 spore sketches of six specimens collected from Réunion and the Seychelles. Proper spores are 10–16 × 8–12 μm long, regularly subglobose to short-ellipsoid. Perispores are 21–35 × 13–20 μm long, often 1–2.5 μm thick and mostly even, with short acicular, often curved apical extensions, mostly 2–5 μm long, occasionally 10 μm long. Among the perispores studied from the three Mare Longue collections from Réunion, 80% were of this type, whereas only 40% of the three Schumm & Frahm collections from Réunion and the Seychelles were of this category, some also with uneven central parts of the perispores. The remaining perispores were thick, irregularly gibbose, occasionally up to 6 μm thick, without any structures developed in regular apical positions. Figure 5D shows spores from the collection Elvebakk 05:694 from New Caledonia. The frequency of acicular perispores is lower and proper spores are shorter, but there is overlap with the spores studied from islands in the Indian Ocean.
Discussion
Gibbosporina cyanea is remarkable in being a cyanolichen within a genus so far known only to include tripartite species dominated by the chlorobiont. Its thick but still heteromerous medulla of chain-forming Nostoc explains why its surface is wrinkled when dry, changing to gelatinous when moist, similar to several species of Pannariaceae and Collemataceae members in general. This might explain why the specimen was determined and published as Leptogium cyanescens Nyl. by Leighton (Reference Leighton1871). The marginal phyllidia look quite similar to the cephalodia of other Gibbosporina species in being pale and bluish grey-brown, contrasting the uniform, brown colour of the main thallus (Figs 1B & 2D). However, this is because the upper cortex of the phyllidia of G. cyanea is much thinner than in the main thallus, making the phyllidia brittle, thus probably enhancing their functioning as vegetative propagules. Still, they are ramifications of the major thallus and not simply partially attached to it, like cephalodia in tripartite Gibbosporina species.
Gibbosporina cyanea is compared with other Gibbosporina species in Table 1, with thalli of G. boninensis, G. bifrons and Leightoniella zeylanensis in Fig. 2, and with ascospores of G. mascarena and G. sphaerospora in Fig. 3. The latter illustrations originate from the spore sketches used by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016). The selected Gibbosporina species in Table 1 all have mini-foliose to foliose cephalodia, and in three of the species the cephalodia are frequently rhizinate, indicating the potential for a free-living state. The spores are most similar to those of G. sphaerospora, a species which is widely distributed in SE Asia and NE Australia and which has broader, black pycnidia. The overall similarity appears to be closer to G. sphaerospora than to G. mascarena, the latter known from Sri Lanka, although this observation can only be verified by future molecular studies. So far, no Gibbosporina specimens appear to have been collected in Sri Lanka during the last 150 years.
Table 1. A comparison of characters of the bipartite Gibbosporina cyanea vs selected tripartite Gibbosporina species. Character states of the tripartite species from Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016).

Magain & Sérusiaux (Reference Magain and Sérusiaux2014) showed that photobiont switches and cephalodia emancipation followed by divergence are evolutionary drivers in Pannariaceae, and in the Pannaria group of genera a tripartite morphology was postulated to be the most likely ancestral state. As understood today, Pannaria consists of 30% tripartite species, with the remaining ones being cyanolichens. In most cases the morphology of the dominating morphs within these two groups is similar. Furthermore, Nostoc phylotypes in cyanospecies of Pannaria, are different to those in cephalodia of tripartites (Magain & Sérusiaux Reference Magain and Sérusiaux2014). This could suggest that photobiont switch rather than cephalodium emancipation was a more likely driver of this evolutionary development.
In the case of Pannariaceae, Magain & Sérusiaux (Reference Magain and Sérusiaux2014) proposed that only photobiont switch occurred between the two major phototypes of cyanolichen. Fuscopannaria viridescens P. M. Jørg. & Zhurb., the only tripartite species in a large cyanolichen genus (Jørgensen & Zhurbenko Reference Jørgensen and Zhurbenko2002; Nelson & Wheeler Reference Nelson and Wheeler2013), could be postulated to have been derived from photobiont switch, as also proposed to have taken place in the genus Peltigera (Miadlikowska et al. Reference Miadlikowska, Kauff, Hofstetter, Fraker, Grube, Hafellner, Reeb, Hodkinson, Kukwa and Lücking2006). Another remarkable example is from Gibbosporina didyma Elvebakk et al., where cephalodia form small chlorobiont thalli which produce apothecia, almost like the adverse situation of a cephalodium on a green thallus (Elvebakk et al. Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016). This transition from cyanomorph to chloromorph is hard to explain, except that the cephalodium apparently cannot produce apothecia itself.
In Gibbosporina, cephalodia emancipation appears more likely. Magain & Sérusiaux (Reference Magain and Sérusiaux2014) analyzed one mycobiont sample of G. mascarena, then named ‘Pannaria tripartite R969’, and included it as a member of the ‘Physma group’, a major clade within their phylogenetic study of Pannariaceae. This group is dominated by cyanospecies, but the cyanobiont of the genus Physma is genetically almost identical to a cephalodiate Nostoc of their analyzed sample of G. mascarena, together forming ‘Nostoc Phylotype E’. Their hypothesis is, therefore, that Physma has originated through emancipation of Gibbosporina cephalodia followed by divergence.
Gibbosporina is an old genus and diverged from Physma at 75 Ma, although this is a cautious estimate (Elvebakk et al. Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016). Another genus, Xanthopsoroma, was described with two austral species (Elvebakk et al. Reference Elvebakk, Robertsen, Park and Hong2010). Apart from the major clade of parmelielloid genera (i.e. containing the genus Parmeliella), Gibbosporina occupies a basal position in recent Pannariaceae phylogenies (Elvebakk et al. Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2020; Spribille et al. Reference Spribille, Fryday, Pérez-Ortega, Svensson, Tønsberg, Ekman, Holien, Resl, Schneider and Stabentheiner2020). It is tripartite, and its isolated position as a basal group to the remaining members of their Physma group was one reason why Magain & Sérusiaux (Reference Magain and Sérusiaux2014) hypothesized that the ancient state of Physma was most likely tripartite.
Several Gibbosporina species have foliose cephalodia superficially resembling cyanospecies within the Physma clade, such as Leightoniella zeylanensis (Fig. 2C), although the latter is genetically distinct (Weerakoon et al. Reference Weerakoon, Aptroot, Wedin and Ekman2018). The proposed long evolutionary history of Gibbosporina, and its dominant and probably ancestral tripartite structure, suggests that Gibbosporina cyanea represents a relatively recent speciation. Its phyllidia have maintained the brittle character and thin cortex of its putative ancestor, and its function as a vegetative dispersal unit, and could be considered homologous to the small-foliose lobules of cephalodia of a tripartite ancestral species, although it has diverged in several characters compared with tripartite Gibbosporina species (Table 1). Such patterns are not known among other members of the Physma clade, except for a possible transitionary state in Lepidocollema polyphyllinum, a cyanolichen which like Gibbosporina cyanea produces smaller lobules, probably also serving as vegetative propagules. Elvebakk et al. (Reference Elvebakk2016) showed that there is a morphological distinction between the cyanobiont of the cephalodium-like lobules versus those of the main thallus, a difference which is not evident in G. cyanea, although these morphotypes have not been compared genetically.
A comparison of Gibbosporina cyanea with related palaeotropical cyanolichen genera within Pannariaceae is summarized in the key below. The taxa included here are primarily divided by perispores being either gibbose, verrucose or smooth. A challenge in comparing G. cyanea with related Pannariaceae genera is that two of the latter, Lepidocollema and Physma, are not monophyletic according to recent phylogenies (Magain & Sérusiaux Reference Magain and Sérusiaux2014; Elvebakk et al. Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016; Rangsiruji et al. Reference Rangsiruji, Boonpragob, Mongkolsuk, Sodamuk, Buaruang, Binchai, Lumbsch and Parnmen2016). The approach here was to focus on specimens already included in phylogenies and to name their clades Lepidocollema I and II and Physma I and II. Perispores from species of these clades are illustrated here while taxonomic conclusions are left for future studies.
Species of Gibbosporina, Physma I and Physma II all share gibbose perispores. Apical perispore extensions are absent in all Gibbosporina species but are present in both Physma I and Physma II. Species of the genus Physma s. lat. were positioned in two paraphyletic clades by Magain & Sérusiaux (Reference Magain and Sérusiaux2014) and in three polyphyletic clades by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016). Two species in two different clades are from Réunion but have unreliable identifications, as do many other Physma species represented in recent phylograms, as indicated by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016) and the present study.
‘Physma I’ is defined here as the clades in both studies containing the sample Jones s. n. of ‘Physma byrsaeum’ from Tahiti, ‘P. byrsaeum R1121’ from Réunion in Magain & Sérusiaux (Reference Magain and Sérusiaux2014) and ‘P. byrsaeum NK-273’ also from Réunion in Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016). Both the latter and Jones s. n. have been studied here (Fig. 5) and show gibbose perispores with wide apical extensions, and additionally with long apiculi in NK-273. It is most likely that the identifications of these specimens, as well as R1121 by Magain & Sérusiaux (Reference Magain and Sérusiaux2014), are incorrect. Hue (Reference Hue1906) reported P. boryanum from Réunion, and his perispore descriptions (the first ones of this species) correspond to those shown for NK-273 here (Fig. 5A). If this determination can be confirmed from type studies, then ‘Physma I’ might represent Physma s. str., since P. boryanum is the generitype of the genus. NK-273 is referred to here as P. cf. boryanum, and the sample Jones s. n. is named Physma sp. These two species have very distinct perispores and their clade Physma I formed a well-supported sister group to all remaining specimens of Physma s. lat., Lepidocollema and Gibbosporina in the phylogram by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016).
Specimens from ‘Physma II’ are shown here to have a considerably high percentage of thick, more even perispores with short, filiform apiculi and fewer, but distinct, gibbae (Fig. 5C & D). One of these illustrated spores lacks gibbae, as do those of P. byrsaeum illustrated by Schumm & Aptroot (Reference Schumm and Aptroot2010). This species is the only widespread pantropical Physma species (according to, for example, GBIF), and was listed as the only one occurring in Réunion by van den Boom et al. (Reference van den Boom, Brand, Ertz, Kalb, Magain, Masson, Schiefelbein, Sipman and Sérusiaux2011). Three specimens collected by Schumm & Frahm in the Seychelles and in Réunion as P. byrsaeum, matching the species concept in Schumm & Aptroot (Reference Schumm and Aptroot2010), Magain & Sérusiaux (Reference Magain and Sérusiaux2014) and Diederich et al. (Reference Diederich, Lücking, Aptroot, Sipman, Braun, Ahti and Ertz2017), have been examined for the present study and correspond to specimens identified as ‘P. radians’ by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016). These identifications are corrected to P. byrsaeum here. The Physma sp. from New Caledonia, analyzed as NK-162 by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016), has the same kind of spores and is also identified as P. byrsaeum here, although a higher percentage of the perispores lack apical extensions and therefore resemble Gibbosporina spores. Nevertheless, the short and filiform diagnostic apical extensions are present, and the specimen was positioned close to the specimens now determined as P. byrsaeum in the phylogram by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016). Their clade of P. byrsaeum, here named ‘Physma II’, is shown as a well-supported clade by Elvebakk et al. (Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016) but is closer to Gibbosporina than ‘Physma I’. ‘Physma II’ needs a different genus affiliation, and the name Dichodium has already been proposed for P. byrsaeum by Nylander (Reference Nylander1888), a topic to be investigated further.
A third Physma clade shown by Elvebakk et al. (2016) included a sample of Physma radians Vain. from Japan and one of P. pseudoisidiatum Aptroot & Sipman from the USA, both first analyzed phylogenetically by Wedin et al. (Reference Wedin, Wiklund, Jørgensen and Ekman2009). Specimens from this clade have not been studied further here.
The genus Pannaria is characterized by verrucose perispores in the key below. Perispore structures are incompletely known for many species of this genus, now being studied by the current author, including reviews of the P. rubiginosa and P. lurida groups. Two representatives from India and Sri Lanka characterizing these groups are included here, with perispore illustrations shown in Fig. 4D and 4E. The studied sample of P. lurida from Sri Lanka deviates from other samples of the species but has its characteristic nodulose apical perispore extensions. The only sample of P. lurida accepted from India by Upreti et al. (Reference Upreti, Divakar and Nayaka2005) is from the Andaman Islands and is described as apiculate on both ends. Both these collections indicate a need to restudy P. lurida in India and Sri Lanka.
The genera Leightoniella and Lepidocollema share smooth and even perispores with gradual and often poorly defined pulvinate apical extensions, which are sometimes absent. The monotypic genus Leightoniella has a perispore described by Weerakoon et al. (Reference Weerakoon, Aptroot, Wedin and Ekman2018) as ‘thick and gelatinous with pointed ends’, matching its illustration as lenticular by Leighton (Reference Leighton1871, fig. 36:1). Personal studies of Leightoniella zeylanensis (Fig. 4A) show a smooth, almost ellipsoid perispore with swollen and obtuse ends, corresponding to the structure of the lower part of the spore illustrated by Weerakoon et al. (Reference Weerakoon, Aptroot, Wedin and Ekman2018). This structure is quite similar to the perispores of the austral genus Xanthopsoroma (see illustrations by Elvebakk et al. (Reference Elvebakk, Robertsen, Park and Hong2010)), a small genus with isolated phylogenetic positions as referred to above.
Ekman et al. (Reference Ekman, Wedin, Lindblom and Jørgensen2014) included only two specimens of the Lepidocollema marianum group in their phylogeny when they redefined the genus Lepidocollema to include tropical members of Parmeliella with thalline excipuli. Magain & Sérusiaux (Reference Magain and Sérusiaux2014) included 13 Parmeliella specimens of this category in their phylogram, which rendered Lepidocollema polyphyletic. Lepidocollema borbonicum was positioned in a different but neighbouring clade to the nine specimens of the L. marianum group. These were united in a monophyletic clade by Weerakoon et al. (Reference Weerakoon, Aptroot, Wedin and Ekman2018), but here the taxon sampling was aimed at defining the position of Leightoniella. Three specimens of L. polyphyllinum were positioned phylogenetically with the differently positioned members of Parmeliella s. str. by Magain & Sérusiaux (Reference Magain and Sérusiaux2014), indicating that the identity of the specimens may need to be confirmed.
The L. marianum group is defined here as ‘Lepidocollema I’, and has thin perispores with smooth walls which are gradually thickened apically (Fig. 4C), corresponding to the patterns shown by Schumm & Aptroot (Reference Schumm and Aptroot2010). ‘Lepidocollema II’ is represented here by a specimen of L. polyphyllinum from the Solomon Islands, and is the same specimen published by Elvebakk (Reference Elvebakk2016). It has similar perispores to ‘Lepidocollema I’ but without apical expansions (Fig. 4B), although the material is too scarce to reach a general conclusion.
Perispore structure appears to be a very useful character among these Pannariaceae genera, where phylogenies coupled with thorough studies of samples are required to obtain an improved understanding.
A key for determining Gibbosporina cyanea and related palaeotropical cyanolichen groups within Pannariaceae
1 Perispores gibbose2
Perispores verrucose or smooth4
2(1) Perispores without apical extensionsGibbosporina cyanea
Perispores with apical extensions3
3(2) Apical extensions broad, either nodulose to pulvinate or tapering and long-apiculate Physma I (incl. P. cf. boryanum)
Apical extensions narrow, short, filiform and often curved, although absent in some spores Physma II (incl. P. byrsaeum)
4(1) Perispores verrucose5
Perispores smooth6
5(4) Perispores with triangular to long-acuminate apical extensionsPannaria rubiginosa
Perispores with nodulose extensionsPannaria lurida group
6(4) Perispores with distinct pulvinate extensions and proper spores < 8 μm wideLeightoniella zeylanensis
Perispores with very weak and gradual apical extensions or without such structures; proper spores > 8 μm wide7
7(6) Perispores with very weak apical extensions; thalli with radiating laciniae on a very distinct blackish hypo- and prothallusLepidocollema I
Perispores without apical extensions, laciniae different and prothallus not very distinctLepidocollema II
Acknowledgements
Curator G. Weerakoon, Natural History Museum, London, is acknowledged for the opportunity to study the collection from BM initiating this study, and acknowledgements are also extended to the curators of the other cited herbaria for facilitating the study of additional material. Felix Schumm, Wagen, Germany, kindly sent reference material of Physma to P. M. Jørgensen, University of Bergen, Norway, who then kindly made them available to the present author along with a Leightoniella sample borrowed from Japan which is published here. Two anonymous referees provided comments improving the manuscript. Mari Karlstad, the Arctic University Museum of Norway, Tromsø, took the photographs included. Direction des Resources Naturelles, Nouméa, kindly gave permission to collect in New Caledonia, and Parc National de La Réunion and Office National des Forêts, St. Denis, Réunion to collect there.
Author ORCID
Arve Elvebakk, 0000-0002-7682-3797.









