Introduction
Bamboos comprise a diverse group of approximately 1250 species and 75 genera (Scurlock et al. Reference Scurlock, Dayton and Hames2000, Yuen et al. Reference Yuen, Fung and Ziegler2017). Bamboos are distributed over approximately 31.5 million ha worldwide (FAO, 2010) and about 80% are indigenous to the Asia–Pacific region, mainly in subtropical to tropical areas (Lobovikov et al. Reference Lobovikov, Schoene and Lou2012). Bamboos provide numerous ecosystem services, such as prevention of landslides and soil erosion, improvement of soil fertility, water conservation, carbon sequestration, and the provision of food and construction materials (Ben-zhi et al. Reference Ben-zhi2005, Lobovikov et al. Reference Lobovikov, Schoene and Lou2012, Scurlock et al. Reference Scurlock, Dayton and Hames2000, Yuen et al. Reference Yuen, Fung and Ziegler2017), although the expansion of bamboo gives negative impacts on forest trees, such as the erosion of tree biodiversity and the delay of tree regeneration (Larpkern et al. Reference Larpkern, Moe and Totland2011). Despite the significant roles played by bamboos in a forest ecosystem, certain ecological characteristics of bamboo species, such as regeneration processes, flowering ecology, and habitat preference, remain poorly understood.
Bamboos are important components of forests in Myanmar. The area of forest occupied by bamboos is 27.85 million ha, representing 84% of the total forested area of 33.32 million ha in Myanmar (FAO, 2015). Bamboos are important resources for human communities in Myanmar, who utilise bamboos for housing materials, materials for daily necessities such as hats, mats and baskets, and food (ITTO, 2007, Rao et al. Reference Rao, Rao and Williams1998). In addition to domestic consumption, bamboo products are sold at markets as an additional source of income.
The size of bamboo populations in Myanmar forests is mainly influenced by two anthropogenic factors. The first factor is disturbance caused by human activities. Bamboos dominate at early to intermediate stages of succession in forest (Lessard and Chouinard, Reference Lessard and Chouinard1980, Rao and Ramakrishnan, Reference Rao and Ramakrishnan1987). Therefore, if an old-growth forest is disturbed by human activities and is transformed into an early successional forest, bamboo populations will expand (Franklin et al. Reference Franklin, Prior, Hogarth and McMahon2010, Gagnon and Platt, Reference Gagnon and Platt2008, Gagnon et al. Reference Gagnon, Platt and Moser2007, Guilherme et al. Reference Guilherme, Oliveira, Appolinario and Bearzoti2004, Söderström and Calderon, Reference Söderström and Calderon1979, Zaczek et al. Reference Zaczek, Baer and Dalzotto2010). The second factor is excessive exploitation of bamboos by local residents, causing a reduction in bamboo population size. These two factors usually coincide in Myanmar forests, including at the present study site at Ottarathiri Township,Naypyidaw Union Territory, Myanmar. Thus, fluctuation in bamboo population size is determined by the balance between these factors.
Changes in bamboo population size may have serious consequences for the forest environment and livelihood of local inhabitants. For example, bamboo expansion may negatively affect forest trees. It may result in lower tree-species diversity in a forest (Larpkern et al. Reference Larpkern, Moe and Totland2011) and may delay forest regeneration after a disturbance event by suppressing seed germination and survival of tree seedlings (Budke et al. Reference Budke, Alberti, Zanardi, Baratto and Zanin2010, Griscom and Ashton, Reference Griscom and Ashton2003, Larpkern et al. Reference Larpkern, Moe and Totland2011, Montti et al. Reference Montti, Campanello, Gatti, Blundo, Austin, Sala and Goldstein2011, Nakashizuka, Reference Nakashizuka1988). However, it may enable improved accessibility to bamboo resources for the local community. Conversely, decrease in the bamboo population size may negatively impact the livelihoods of local residents by limiting the availability of bamboo resources. Therefore, sustainable management of bamboo populations is of paramount importance in rural areas of Myanmar. For implementation of appropriate and sustainable management practices, ecological information on the habitat preferences of bamboos is vital because it will enable the creation of suitable sites in forests to enhance bamboo populations.
In forests, the spatial distribution of trees and bamboos is influenced by biotic and abiotic factors. When considering biotic factors, the spatial relationship of a tree species with other tree species often affects the spatial distribution of trees in a forest (Masaki et al. Reference Masaki, Suzuki, Niiyama, Iida, Tanaka and Nakashizuka1992, Yamada and Suzuki, Reference Yamada and Suzuki1997). For example, shade-intolerant short-stature trees are usually clumped in canopy gaps and avoid large trees because they cannot survive in dense shade. Consequently, such species may not distribute with large trees. In Myanmar forests, bamboos are sub-canopy or understorey species and are fast-growing, early successional species (Gadgil and Prasad, Reference Gadgil and Prasad1984, Lessard and Chouinard, Reference Lessard and Chouinard1980, Rao and Ramakrishnan, Reference Rao and Ramakrishnan1987, Söderström and Calderon, Reference Söderström and Calderon1979). Hence, we hypothesise that bamboos are spatially separated from large trees to avoid the direct shade that is created by the large trees.
Abiotic factors are also important determinants of the spatial structure of forests. The soil water and nutrient availabilities are distributed heterogeneously in a forest and are strongly associated with topography (Famiglietti et al. Reference Famiglietti, Rudnicki and Rodell1998). For example, ridge sites tend to be drier and more nutrient deficient than valley sites (Hirai et al. Reference Hirai, Matsumura, Hirotani, Sakurai, Ogino and Lee1997, Ishizuka et al. Reference Ishizuka, Tanaka, Sakurai, Hirai, Hirotani, Ogino, Lee and Kendawang1998, Palmiotto, Reference Palmiotto1998, Tan et al. Reference Tan, Yamakura, Tani, Palmiotto, Mamit, Pin, Davies, Ashton and Baillie2009, Tateno and Takeda, Reference Tateno and Takeda2003, Yamada et al. Reference Yamada, Tomita, Itoh, Yamakura, Ohkubo, Kanzaki, Tan and Ashton2006). Tree species often require a specific combination of resources, resulting in an association of trees with topography (Bazzaz, Reference Bazzaz1991, Sork et al. Reference Sork, Bramble and Sexton1993, Whitmore, Reference Whitmore1990). Habitat associations of trees in relation to topography have been repeatedly observed among tropical tree species (Davies et al. Reference Davies, Palmiotto, Ashton, Lee and Lafrankie1998, Debski et al. Reference Debski, Burslem, Palmiotto, Lafrankie, Lee and Manokaran2002, Harms et al. Reference Harms, Condit, Hubbell and Foster2001, Hubbell and Foster, Reference Hubbell, Foster, Diamond and Case1986, Marod et al. Reference Marod, Hermhuk, Sungkaew, Thinkampheang, Kamyo and Nuipakdee2019, Marod et al. Reference Marod, Phumphuang and Wachrinrat2021, Sri-Ngernyuang et al. Reference Sri-Ngernyuang, Kanzaki, Mizuno, Noguchi, Teejuntuk, Sungpalee, Hara, Yamakura, Sahunalu, Dhanmanonda and Bunyavejchewin2003, Svenning, Reference Svenning1999, Webb and Peart, Reference Webb and Peart2000, Yamada et al. Reference Yamada, Itoh, Kanzaki, Yamakura, Suzuki and Ashton2000, Yamada et al. Reference Yamada, Noor and Okuda2010, Yamada et al. Reference Yamada, Oka and Suzuki2007, Yamada et al. Reference Yamada, Tomita, Itoh, Yamakura, Ohkubo, Kanzaki, Tan and Ashton2006). Therefore, the distribution of bamboo may be highly influenced by topography (Hayashi and Yamada, Reference Hayashi and Yamada2008). However, no previous study has verified this influence in Myanmar and limited information is available on the relationship between bamboos distribution and topography (Tanaka et al. Reference Tanaka, Marod, Ishida, Takahash, Saitoh and Nakashizuka2010).
The present study was conducted in a commercial tree plantation forest established in a mixed deciduous forest in Ottarathiri Township, Naypyitaw Union Territory, Myanmar. In total, three bamboo species (Cephalostachyum pergracile Munro, Bambusa polymorpha Munro, and Dinochloa macclellandii (Munro) Kurz.) were present. We examined the spatial distribution of the three bamboo species with specific reference to the distribution of large trees and topography. First, we assessed whether the bamboo species were not distributed with large trees. Next, we examined the relationship between the distribution of these bamboos and topography. If a bamboo species showed a preference for a specific topographic condition, the following two premises should be met: (1) bamboos are heterogeneously distributed in accordance with the heterogeneous distribution of topographic conditions in a forest and (2) bamboos are more concentrated at a site with a specific topographic condition. We therefore explored whether these premises were met for the three bamboo species. In addition, assuming that bamboo species prefer a site with specific topographic conditions, we assessed the suitable topography for each species. However, adopting this approach, we aimed to obtain information on the topographic site where a bamboo species appears more than the other topographic site (i.e., habitat requirements of the bamboo species) to enhance the management of bamboo populations in Myanmar forests.
Method
Study site
The study was conducted in a commercial tree plantation forest established in a mixed deciduous forest in the Bago Mountains, Ottarathiri Township, Naypyitaw Union Territory, Myanmar (ca. 200 m a.s.l.), in 2016. The study site is located in a tropical monsoon climatic zone with a distinct rainy season (from mid-May to mid-October) and a dry season (from mid-February to April). The average annual temperature is 24.5°C and average annual precipitation is 1421.1 mm with 97% falling in the rainy season (Moe Swe Forest Research Center, unpublished data; the average monthly temperature and precipitation are shown in Figure S1). The natural vegetation at the study site was dominated by broad-leaved deciduous trees, such as Tectona grandis L.f., Pterocarpus macrocarpus Kurz., Markhamia stipulata (Wall.) Seem., and Xylia xylocarpa (Roxb.) W.Theob. Three bamboo species, C. pergracile, B. polymorpha, and D. maclellandii, were present in the forest. Tectona grandis can attain a diameter at breast height (DBH) of 60 cm in old-growth forests in the study site. The forest canopy height of old-growth forest was about 25 m. The forest canopy was not closed because some canopy trees had been cut by local residents. The local community obtains forest products from the forest for daily use, and scars from the logging of trees and cutting of bamboos were observed.
We established an 80 m × 110 m (0.88 ha) plot, which was divided into 88 quadrats, each 10 m × 10 m, within a commercial tree plantation (T. grandis, X. xylocarpa, P. macrocarpus, and Millettia tetraptera Kurz.) established in 1978 by the Myanmar Government. Planting intervals between trees were about 2.5 m. Prior to the establishment of the plantation, the site was cut and burned. Weeding was conducted several times until 2 years after the establishment of the plantation. Bamboos might have been able to survive these treatments because they are tolerant of fire and other disturbances (Kachina et al. Reference Kachina, Kurokawa, Oguro, Nakashizuka, Tanaka, Thinkampheang, Sungkaew, Panuthai and Marod2017, Tanaka et al. Reference Tanaka, Marod, Ishida, Takahash, Saitoh and Nakashizuka2010). No tree-planting activities have been conducted subsequently; therefore, all commercial trees were planted in 1978. In this forest, naturally regenerating trees, which emerged after the plantation establishment, were observed. Thus, all trees growing in the plot originated during or after the establishment of the plantation. The height of the forest was approximately 25 m. The largest tree had attained DBH of approximately 50 cm. The forest floor was burnt annually by local residents in the dry season to manage forest understorey. Forest fire may delay forest tree regeneration while enhancing the production of bamboo shoots to eat and the growth of some useful understorey grasses for thatch.
A topographic survey of the plot was conducted to generate an elevational contour map. The height difference between the highest and lowest points in the plot was 15 m. By using the slope angle of quadrats, we classified the 88 quadrats classified as either steep slope (slope angle >20°) or gentle slope (including gentle ridge, slope angle ≤20°). The plot did not include any valley bottoms. We identified 9 steep slope quadrats and 79 gentle slope quadrats (Figure S2).
Study species
The three bamboo species studied were deciduous that shed leaves in dry seasons. McClure (Reference McClure1993) distinguished two distinct rhizome patterns among bamboo species, namely leptomorphic and pachymorphic rhizomes. A leptomorphic rhizome (a horizontally spreading rhizome) bears solitary culms that are evenly spaced between culms, resulting in a large clonal colony with scattered culms. Temperate bamboo species, such as Phyllostachys heterocycla (Carrière) Mitford, P. bambusoides Siebold et Zucc., and P. nigra (Lodd. et Lindl.) Munro var. henonis (Mitford) Stapf ex Rendle, often develop leptomorphic rhizomes. In contrast, pachymorphic rhizomes lack leptomorphic rhizomes and show limited horizontal spread, resulting in dense aggregated clumps of culms (Figures S3a and S4). Many tropical bamboo species exhibit the latter rhizome pattern, including the three bamboo species in the present study. As a result, we could separate each clump spatially from another as an apparent genet.
At the study site, the tallest bamboo species, C. pergracile, seldom attained a height of 16 m (Figure S5). Culm diameters were always less than 7 cm. Bambusa polymorpha was the second-tallest species and was always shorter than 15 m in height (Figure S5). The maximum attainable culm diameter was 10 cm. Dinochloa maclellandii was the shortest bamboo species and never grew taller than 9 m (Figure S5). Culm diameters were always less than 7 cm. The sizes of bamboos in the study plot were identical to those found in old-growth forests around the study plot.
The bamboo species are monocarpic and sexually reproduce only once in their life and die soon after sexual reproduction. Some conspecific clumps in a local area often bloom simultaneously (Janzen, Reference Janzen1976, Makita et al. Reference Makita, Konno, Fujita, Takada and Hamabata1993, Söderström and Calderon, Reference Söderström and Calderon1979, Zheng et al. Reference Zheng, Lin, Fu, Wan and Ding2020), resulting in sporadic to mass flowering in the region (Janzen, Reference Janzen1976, Kharlyngdoh et al. Reference Kharlyngdoh, Sahoo, Shukla, Devi, Kumar, Hamdy and Khalkho2021, Marod D et al. Reference Marod, Kutintara, Yarwudhi, Tanaka and Nakashisuka1999, Takeda, Reference Takeda2019). The flowering interval of B. polymorpha is 60 years, whereas those of C. pergracile and D. maclellandii are still unknown (Zheng et al. Reference Zheng, Lin, Fu, Wan and Ding2020). The most recent flowering event for C. pergracile occurred in 1997 at the study site (Nyan Htun, Forest Research Institute Myanmar, personal communication). There is no record of the most recent sporadic flowering event for B. polymorpha and D. maclellandii, although it is known that B. polymorpha has not flowered after 1997 (Nyan Htun, Forest Research Institute Myanmar, personal communication).
Cephalostachyum pergracile is utilised by the local community for housing materials and materials for daily necessities, such as baskets and hats. Bambusa polymorpha shoots are edible and consumed for food, whereas D. maclellandii is not utilised for any purpose (ITTO, 2007, Kharlyngdoh et al. Reference Kharlyngdoh, Sahoo, Shukla, Devi, Kumar, Hamdy and Khalkho2021, Rao et al. Reference Rao, Rao and Williams1998).
Field methods
We measured the heights of 27 trees, 20 C. pergracile, 10 B. polymorpha, and 10 D. maclellandii individuals to examine the allometric relationship between DBH and height (Figure S5). These samples ranged from the smallest to the largest individuals.
Typically, a bamboo seedling develops only a few, annual culms less than 2 cm in diameter in a clump. Subsequently, it eventually produces perennial culms more than 2 cm in diameter. We mapped bamboo clumps with a perennial culm greater than 2 cm in diameter and identified their species. The number of culms in a clump was also counted. We measured the perimeter of a polygon formed by culms in a clump at breast height (Figure S3b). By approximating the polygon to a circle with the same circumference, the perimeter of the polygon was converted into the diameter of the circle. We termed this diameter the ‘clump diameter’.
Immature Bambusa tulda Roxb. plants in Thailand show little growth under suppression by other bamboos or trees but promptly produce rapid growth after removal of the suppression (Tanaka et al. Reference Tanaka, Marod, Ishida, Takahash, Saitoh and Nakashizuka2010). Because we were interested in the suitable conditions for growth and regeneration of bamboos, we focused only on the distribution of large (mature) clumps. Therefore, we used only large bamboo clumps for analysis of spatial distributions. We assumed that clumps larger than 50 cm in clump diameter were large and mature for C. pergracile and B. polymorpha, and those clumps larger than 20 cm in diameter for D. maclellandii (Figure S6). These clumps usually had a culm that attained the maximum attainable height of the species.
In this study, trees were classified into two size classes, large and small, in relation to bamboo heights in the plot. Because the tallest bamboo in the plot was approximately 16 m (Figure S5), trees taller than 16 m were categorised as ‘large’. In the forest, bamboos were never taller than the large trees. The other trees were categorised as ‘small’. According to the relationship between DBH and height for trees (Figure S7), the 16 m height of trees approximately coincided with 20 cm DBH. Therefore, trees larger than 20 cm DBH were classified as ‘large’. Within the plot, we mapped large trees (DBH > 20 cm) and identified them to species.
Spatial patterns and spatial relationship between bamboos
The spatial patterns of the bamboo species were analysed using Besag’s L(t) function (Besag, Reference Besag1977) and a square-root transformation of Ripley’s K(t) function (Ripley, Reference Ripley1977). A value of L(t) = 0 indicates that the spatial distribution of a given bamboo species is random. A value of L(t) > 0 indicates a clumped (aggregated) distribution, and a value of L(t) < 0 indicates a uniform distribution.
The spatial relationship between pairs of bamboo species was analysed using L 12(t), a square-root transformation of the K 12(t) function (Lotwick and Silverman, Reference Lotwick and Silverman1982), which is a modification of Ripley’s K(t) for bivariate data (Fortin and Dale, Reference Fortin and Dale2005). A value of L 12(t) = 0 indicates that the spatial distributions of the two bamboo species are spatially independent of each other. A value of L 12(t) > 0 indicates that the spatial distributions of the two bamboo species are attraction between them (a species tends to distribute with the counterpart species). Hereafter, this is referred to as a positive association between them. Lastly, a value of L 12(t) < 0 indicates that the spatial distributions of the two bamboo species are repulsion between them (each species tends to exclusively distribute from the counterpart species). Hereafter, this is referred to as a negative association. The significance of both L(t) and L 12(t) was determined by Monte Carlo simulations (Besag, Reference Besag1977, Besag and Diggle, Reference Besag and Diggle1977, Marriott, Reference Marriott1979, Yamasaki et al. Reference Yamasaki, Yamada and Okuda2013). For analysis of the spatial pattern of bamboos (L(t)), the null hypothesis is complete spatial randomness, whereas the null hypothesis for the spatial relationship between two tree species (L 12(t)) is spatial independence of their distributions. The 95% confidence envelopes for L(t) were obtained from 5000 permutations of random points of the same number of bamboos analysed, and those for L 12(t) were obtained by 5000 permutations of torus-translation shifts of the spatial distribution of one bamboo species (Diggle, Reference Diggle1983, Yamada et al. Reference Yamada, Tomita, Itoh, Yamakura, Ohkubo, Kanzaki, Tan and Ashton2006, Yamasaki et al. Reference Yamasaki, Yamada and Okuda2013).
In addition, we analysed the spatial relationship between bamboos and large trees or stumps. We examined the spatial relationship of bamboos with large trees because large trees may cast shade on bamboos, making the site unfavourable for bamboo growth. Furthermore, bamboos are expected to be distributed exclusively from large trees. Similarly, bamboos may have a positive spatial relationship with stumps, because bamboo growth is likely to be enhanced by the removal of large trees.
Association of bamboos with topography
The distributional association of bamboos with topography was analysed using Fisher’s exact test for count data. This test revealed only whether a bamboo species is distributed significantly more abundantly on steep or gentle slopes. To determine if bamboos were distributed more abundantly on steep or gentle slopes, we calculated the adjusted density of each bamboo species for steep and gentle slopes by dividing the density of the bamboo species in the focal topography by the average density of the bamboo species across the entire plot (Yamada et al. Reference Yamada, Oka and Suzuki2007, Yamada et al. Reference Yamada, Tomita, Itoh, Yamakura, Ohkubo, Kanzaki, Tan and Ashton2006). Adjusted density values >1 suggest more abundantly than the average across the plot (a positive association between the species and topography), whereas values <1 suggest that the species less abundantly than the average across the plot (a negative association between the species and topography).
Results
The density of trees larger than 20 cm DBH was 111 trees ha−1, and the total basal area was 8.79 m2 ha−1 in the plot (Table S1). Approximately, 62.5% of the trees in the plot were planted trees of T. grandis, X. xylocarpa, P. macrocarpus, and M. tetraptera. We recorded 150 tree stumps of more than 20 cm in diameter in the plot, and 90% of these stumps were of the commercially planted tree species. In total, 489, 76, and 62 clumps of C. pergracile, B. polymorpha, and D. maclellandii, respectively, were recorded in the plot (Table S2). Among these clumps, 219, 62, and 49 clumps were categorised as ‘large’ for C. pergracile, B. polymorpha, and D. maclellandii, respectively. The number of culms per clump increased with clump diameter for all species (Figure S8).
The positions of C. pergracile, B. polymorpha, and D. maclellandii clumps in the plot are shown in Figure 1a–c. The L(t) values for all bamboo species were above the 95% confidence envelopes (Fig. 2a–c), indicating that each of the bamboo species showed a clumped distribution of clumps, which was significantly different from a random distribution.

Figure 1. Spatial distribution of Cephalostachyum pergracile (a; n = 219), Bambusa polymorpha (b; n = 62), and Dinochloa maclellandii (c; n = 49) in an 80 m × 110 m plot in a commercial tree plantation forest on the Bago Mountains, Ottarathiri Township, Naypyitaw Union Territory, Myanmar. The contour interval is 1 m in elevation. Spatial distributions of large trees (DBH > 20 cm) (d) and stumps (e) are also shown.
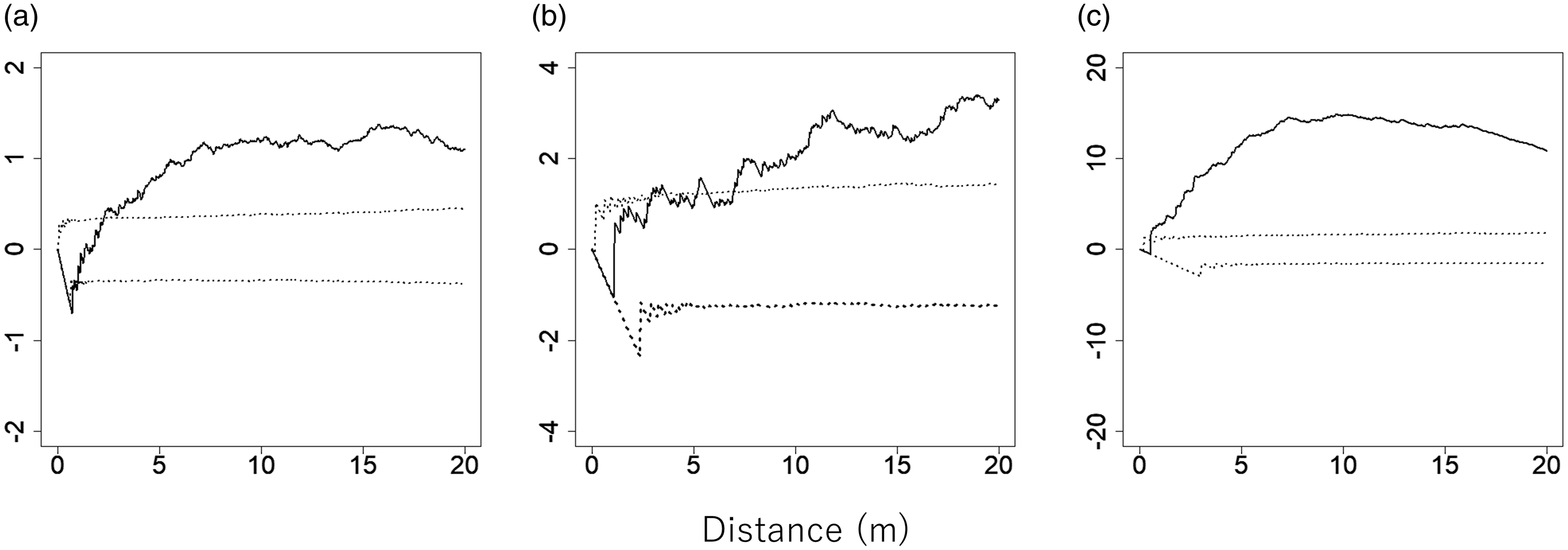
Figure 2. L(t) values for Cephalostachyum pergracile (a; n = 219), Bambusa polymorpha (b; n = 62), and Dinochloa maclellandii (c; n = 49) in an 80 m × 110 m plot in a commercial tree plantation forest on the Bago Mountains, Ottarathiri Township, Naypyitaw Union Territory, Myanmar (solid lines). A value of L(t) = 0, L(t) > 0, and L(t) < 0 indicates a random, clumped, and uniform distribution, respectively. The 95% confidence envelopes for L(t), which were obtained by 5000 permutations of random points of the same number of bamboos, are indicated by dotted lines.
The pair of C. pergracile and D. maclellandii, and that of C. pergracile and B. polymorpha, showed a significant negative spatial association. Hence, the species were exclusively distributed separately from each other in the plot (Figure 3). However, the pair of B. polymorpha and D. maclellandii showed a statistically non-significant spatial relationship with each other.
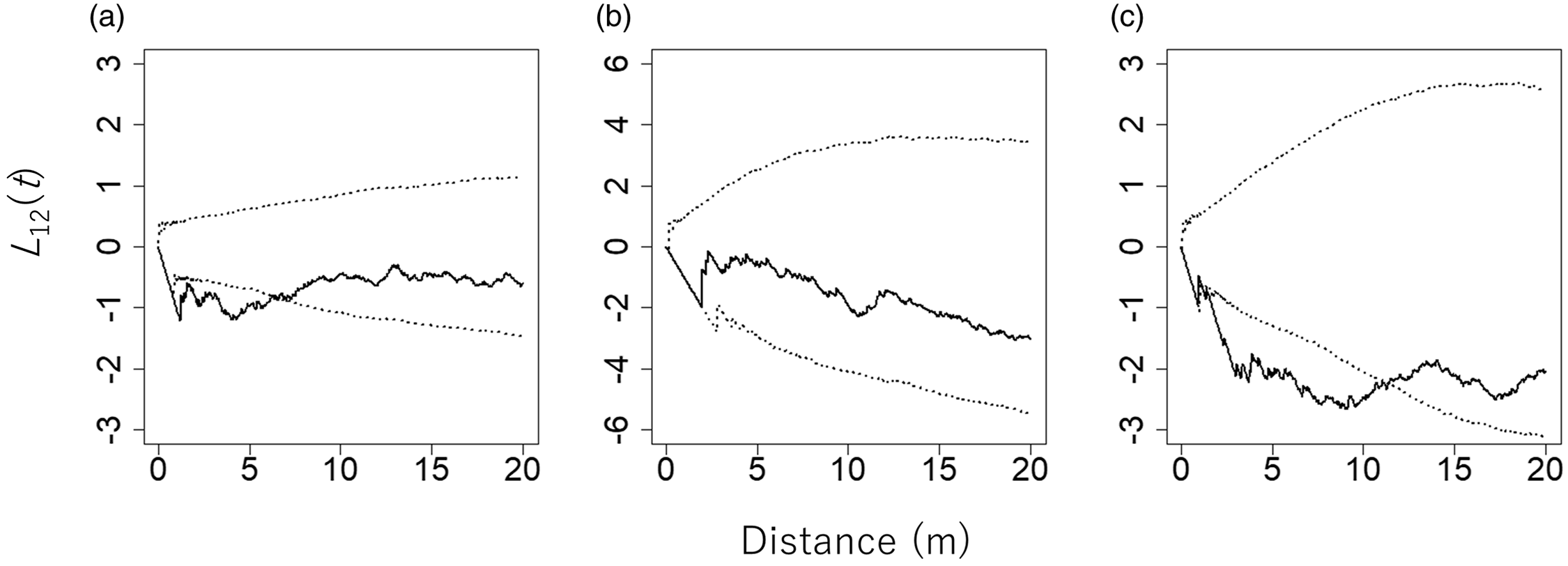
Figure 3. L 12(t) values between pairs of bamboo species in an 80 m × 110 m plot in a commercial tree plantation forest on the Bago Mountains, Ottarathiri Township, Naypyitaw Union Territory, Myanmar (solid lines). The pairs Cephalostachyum pergracile (n = 219) and Bambusa polymorpha (n = 62) (a), B. polymorpha and Dinochloa maclellandii (n = 49) (b), and D. maclellandii and C. pergracile (c) are shown. A value of L 12(t) = 0, L 12(t) > 0, and L 12(t) < 0 indicates that the spatial distributions of the two bamboo species are spatially independent, positively associated (attraction), and negatively associated (repulsion), respectively. The 95% confidence envelopes for L 12(t), which were obtained by 5000 permutations of torus-translation shifts of the spatial distribution of one bamboo species, are indicated by dotted lines.
Cephalostachyum pergracile and D. maclellandii were distributed significantly more abundantly on either gentle slopes or steep slopes (Table 1). However, the nature of the associations was opposite for these two species. Cephalostachyum pergracile was more abundant on gentle slopes. In contrast, D. maclellandii was most abundant on steep slopes and less common on gentle slopes. The adjusted density of D. maclellandii on steep slopes was more than 10 times higher than that on gentle slopes (Table 1), suggesting that this species was strongly associated with steep slopes. Bambusa polymorpha was not distributed significantly more abundantly on neither gentle slopes nor steep slopes. However, the adjusted density of B. polymorpha on gentle slopes was higher than that on steep slopes (Table 1).
Table 1. Association of three bamboo species, large trees and stumps with topography in an 80 m × 110 m plot in a commercial tree plantation forest on the Bago Mountains, Ottarathiri Township, Naypyitaw Union Territory, Myanmar.

+ Values of adjusted density >1.0 and <1.0 indicate that a species shows a positive and negative association with topography, respectively.
Asterisks indicate the significance level from the analysis of the association of bamboo species and topography (count data, Fisher’s exact test; *P < 0.05, ***P < 0.001).
Large trees and stumps were not distributed significantly more abundantly on neither gentle slopes nor steep slopes (Table 1). Analysis of the spatial relationship between bamboos and large trees yielded the following results (Figure 4). Bambusa polymorpha showed a significant negative relationship with large trees, whereas the other two bamboo species did not show such a relationship. Dinochloa maclellandii showed a statistically non-significant spatial relationship with large trees, and C. pergracile had a significant positive spatial relationship with large trees.
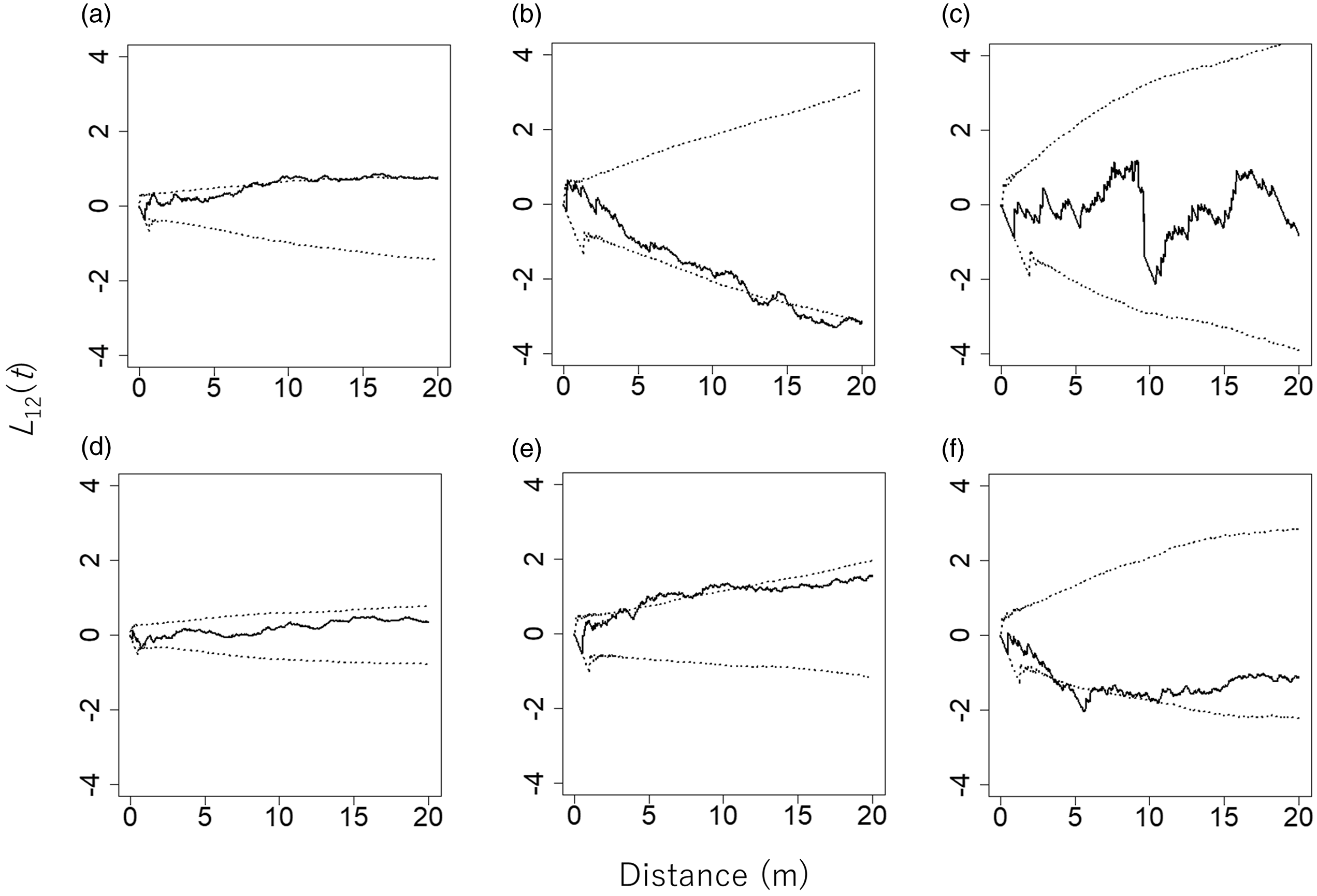
Figure 4. L 12(t) values between pairs of bamboo species and large trees (DBH > 20 cm, n = 96) in an 80 m × 110 m plot in a commercial tree plantation forest on the Bago Mountains, Ottarathiri Township, Naypyitaw Union Territory, Myanmar (solid lines). The pairs Cephalostachyum pergracile and large trees (a), Bambusa polymorpha and large trees (b), and Dinochloa maclellandii and large trees (c) are shown. L 12(t) values between pairs of bamboo species and stumps (DBH > 20 cm, n = 150) are also shown (solid lines). All stumps were the result of logging after establishment of the plantation in 1978. Shown are the pairs Cephalostachyum pergracile and stumps (d), Bambusa polymorpha and stumps (e), and Dinochloa maclellandii and stumps (f). The 95% confidence envelopes are indicated by dotted lines.
Bambusa polymorpha showed a significant positive spatial relationship with stumps (Figure 4), implying that B. polymorpha growth might have been enhanced by the removal of large trees. However, the other two bamboo species differed in their spatial relationship with stumps. A statistically non-significant spatial relationship between C. pergracile and stumps was observed. Dinochloa maclellandii showed a significant negative spatial relationship with stumps, suggesting that the distribution of this bamboo species did not coincide with stumps.
Discussion
If bamboo distribution is associated with topography, the following two premises should be met: (1) bamboos are heterogeneously distributed in a forest in accordance with the heterogeneous distribution of topographic conditions and (2) bamboos are more abundant at a site with a specific topographic condition. In the present study, we examined whether these two premises were supported by the experimental data. The spatial analyses revealed a clumped distribution, which was significantly different from a random distribution for all three bamboo species examined. This suggests that all bamboo species heterogeneously distribute in a forest because they appeared more in one site while distributed less in the other sites. Therefore, the first premise was confirmed to be met for each species.
The second premise was met only for C. pergracile and D. maclellandii. The distribution of C. pergracile tended to be found more abundantly on gentle slopes (including gentle ridges) than on steep slopes. This spatial association pattern of C. pergracile is consistent with the findings of Tanaka et al. (Reference Tanaka, Marod, Ishida, Takahash, Saitoh and Nakashizuka2010), who reported that this species was distributed most frequently on a gentle ridge in a Thailand forest. Thus, the topographic habitat of C. pergracile should include gentle slopes and ridges. In contrast, D. maclellandii was strongly associated with steep slopes that C. pergracile avoided. Hence, steep slopes should be the topographic habitat for D. maclellandii. Bambusa polymorpha was evenly distributed irrespective of topography and thus may not exhibit a habitat preference in terms of topography.
The influence of topography on bamboo distribution is unlikely to be direct. The topography within the plot may be correlated with edaphic variables, such as soil nutrient and soil water availabilities (Hirai et al. Reference Hirai, Matsumura, Hirotani, Sakurai, Ogino and Lee1997, Ishizuka et al. Reference Ishizuka, Tanaka, Sakurai, Hirai, Hirotani, Ogino, Lee and Kendawang1998, Palmiotto, Reference Palmiotto1998, Tateno and Takeda, Reference Tateno and Takeda2003, Yamada et al. Reference Yamada, Tomita, Itoh, Yamakura, Ohkubo, Kanzaki, Tan and Ashton2006). A finer-resolution classification of habitats incorporating such edaphic characteristics in future analyses is highly likely to provide an improved understanding of the habitat association of bamboos.
Cephalostachyum pergracile showed an exclusive spatial relationship with D. maclellandii. This exclusive distribution may be, at least partly, explained by the opposite topographic preferences of the species. However, topography cannot explain the exclusive distributions observed between C. pergracile and B. polymorpha.
The bamboo species we studied were sub-canopy to understorey, fast-growing, early successional species. On this basis, we hypothesised that the species would be spatially separated from large trees to avoid the direct shade cast by the trees. Similarly, we predicted that bamboos would show a positive spatial relationship with stumps because bamboo growth is likely to be enhanced by removal of large trees. The present spatial analyses supported these hypothesised spatial relationships only for B. polymorpha. These results suggest that B. polymorpha is the most shade intolerant among the three bamboo species examined and is less likely to form a large (mature) clump when growing under the canopy of large trees. Release from suppression by large trees may be necessary for the regeneration of B. polymorpha.
In contrast, C. pergracile showed a positive spatial relationship with large trees, suggesting that this species tended to be found near large trees. This finding implies that C. pergracile is more shade tolerant than the other two bamboo species. Differences in shade tolerance between B. polymorpha and C. pergracile may explain the exclusive distribution of the two species. At the present study site, B. polymorpha may dominate in well-lit areas and C. pergracile may dominate in more densely shaded areas, resulting in their exclusive distribution. However, this interpretation fails to explain why C. pergracile did not invade well-lit areas.
The exclusive distribution of B. polymorpha and C. pergracile may not be solely caused by the abovementioned differences in shade tolerance. An additional possibility may be the timing of flowering. Both species flower sporadically (Devi et al. Reference Devi, SHARMA, Singh and Bhattacharyya2014, Marod et al. Reference Marod, Neumrat, Panuthai, Hiroshi and Sahunalu2005, McClure, Reference McClure1993, Suyama et al. Reference Suyama, Suzuki and Makita2010) and some clumps flower simultaneously within a region. Bambusa polymorpha flowers at 60-year intervals (Zheng et al. Reference Zheng, Lin, Fu, Wan and Ding2020), but the flowering interval for C. pergracile is unknown. Both bamboo species are monocarpic and the culms die soon after flowering. The flowering years differ between the species; the most recent flowering event for C. pergracile at the study site was recorded in 1997 and B. polymorpha did not bloom at that time.
Assuming that seedlings of B. polymorpha and C. pergracile must avoid shade from conspecific and heterospecific bamboos to grow into large (mature) plants, they will be unable to establish in a space already occupied by either species at the time of flowering. In other words, provided that the culms of a bamboo species have already occupied a space in a forest at the time of flowering of the other bamboo species, seedlings of the flowering species cannot enter the occupied sites and can only establish in unoccupied spaces. Ultimately, seedlings may be able to regenerate only in canopy gaps left by chance or in well-lit areas following the death of flowering culms. If this is true, it should create an exclusive spatial relationship between the two species. In this manner, occupation of spaces by a species may limit the spaces available for regeneration of the other species. This hypothesis should be tested in a future study by examining the distribution of seedlings and the process of seedling establishment at the next flowering event.
The information on habitat preferences obtained by this study will contribute to suitable bamboo management practices in Myanmar. Bambusa polymorpha, a shade-intolerant bamboo species, may regenerate only in a site where it can intercept sufficient light. A brightly lit understorey environment is crucial to enhance the growth and regeneration of this species. The shade-tolerant C. pergracile may be more dependent on topography than the light environment. The topographic habitats preferred by C. pergracile are gentle slopes or ridges, whereas D. maclellandii regenerates on steep slopes. By providing or managing such habitats, it will be feasible to enhance populations of these two bamboo species in Myanmar forests.
Bamboos cover a wide area of the world (FAO, 2010). Especially in Asian mixed deciduous tropical forests, bamboos are commonly found and are among the important components (Marod et al. Reference Marod, Neumrat, Panuthai, Hiroshi and Sahunalu2005, Kharlyngdoh et al. Reference Kharlyngdoh, Sahoo, Shukla, Devi, Kumar, Hamdy and Khalkho2021). The bamboo species studied were widely distributed in Asia (Marod et al. Reference Marod, Neumrat, Panuthai, Hiroshi and Sahunalu2005, Kachina et al. Reference Kachina, Kurokawa, Oguro, Nakashizuka, Tanaka, Thinkampheang, Sungkaew, Panuthai and Marod2017, Kharlyngdoh et al. Reference Kharlyngdoh, Sahoo, Shukla, Devi, Kumar, Hamdy and Khalkho2021). For example, C. pergracile was commonly found not only in Myanmar forests but also in wide areas from India to Thailand (Marod et al. Reference Marod, Neumrat, Panuthai, Hiroshi and Sahunalu2005, Kharlyngdoh et al. Reference Kharlyngdoh, Sahoo, Shukla, Devi, Kumar, Hamdy and Khalkho2021). Local residents in those areas exploited bamboo resources like people in Myanmar (Kharlyngdoh et al. Reference Kharlyngdoh, Sahoo, Shukla, Devi, Kumar, Hamdy and Khalkho2021). The importance of the information on bamboos’ habitat preferences revealed by this study on the forest management will not be limited to Myanmar forests, and the findings will contribute to the forest management in the other mixed deciduous forests with the bamboos that widely spread over a huge area.
Local residents in our study site used forest fire to manage forest understorey. The fire may delay the regeneration of forest trees, especially fire-intolerant species. Because bamboos can survive forest fire (Kachina et al. Reference Kachina, Kurokawa, Oguro, Nakashizuka, Tanaka, Thinkampheang, Sungkaew, Panuthai and Marod2017), bamboos may obtain competitive advantage over tree species from annual forest fires. Consequently, forest fire may increase the dominance level of bamboos in mixed deciduous forests. Not only in our study site but also a lot of other mixed deciduous forests in Asia were managed by fire (Kachina et al. Reference Kachina, Kurokawa, Oguro, Nakashizuka, Tanaka, Thinkampheang, Sungkaew, Panuthai and Marod2017). Since forest fire may give a big impact on species composition of a forest, for a sound practice of the forest management by fire, more studies to elucidate the roles of fire in the regeneration of bamboos and tree species in mixed deciduous forests are clearly necessary.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0266467423000172
Acknowledgements
We thank M. Miura, K. Ueda, T. Horikane, and M. Kobayashi for helping with fieldwork. We thank Robert McKenzie, PhD, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.
Financial support
This study was financially supported by the Environmental Research and Technology Development Fund (#4-1404 and #1-1903) and by the Japan Society for the Promotion of Sciences (grant no. 20K06827 from 2020 to 2022).
Competing interests
The authors declare none.








