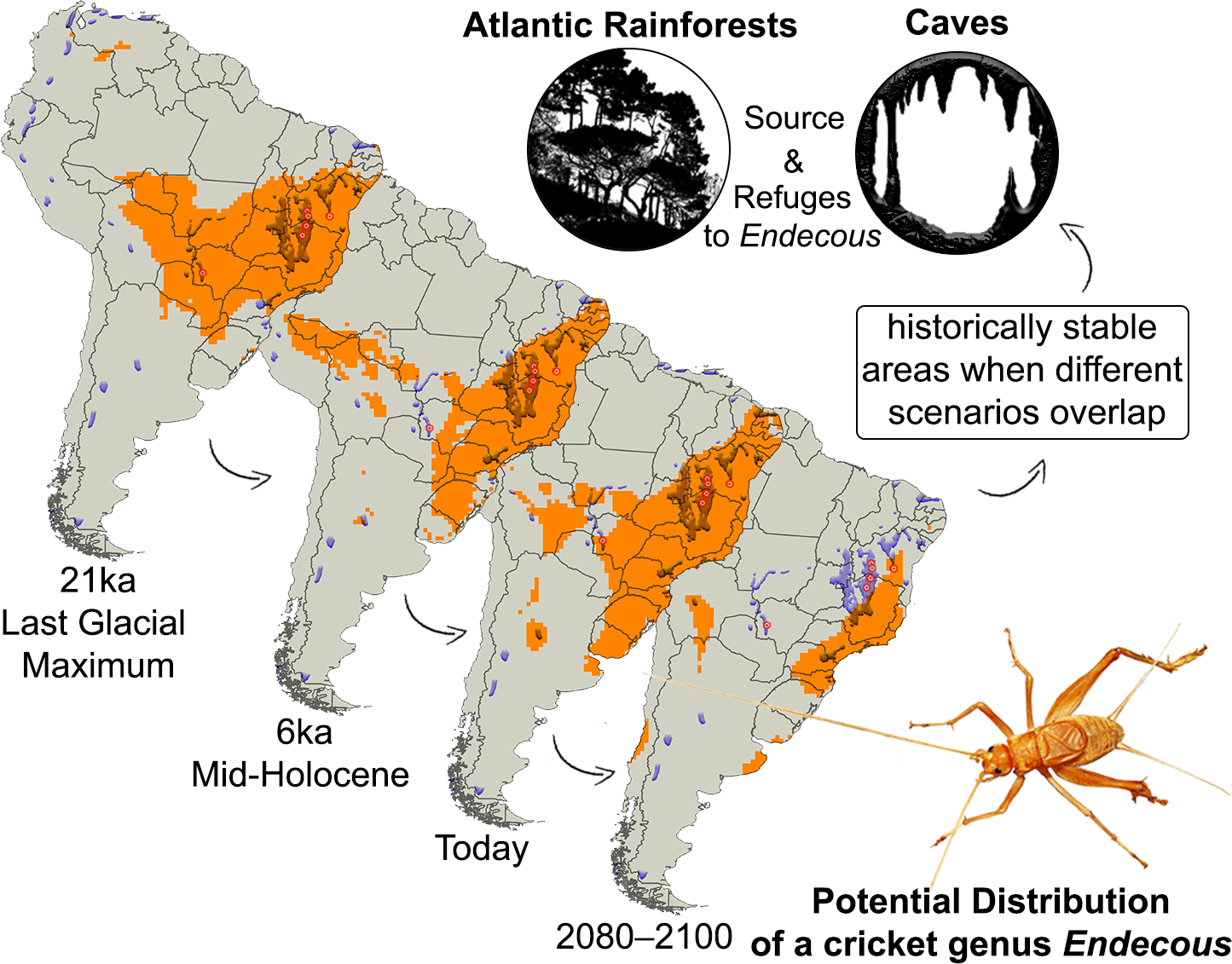
Introduction
Caves comprise natural cavities associated with different types of lithologies, that allow the colonization of different organisms (Moldovan et al. Reference Moldovan, Kováč and Halse2018, White & Culver Reference White and Culver2012). In South America, caves are formed in geological groups dating from the Precambrian (Bolivia and Paraguay), Jurassic, Tertiary (Venezuela) and Cretaceous (areas along the Andes Mountain range, e.g., Bolivia, Colombia, Ecuador, Peru) (Auler Reference Auler and Gunn2004). In Brazil, caves are associated with rocks of different ages, since Paleoproterozoic to Quaternary (Auler et al. Reference Auler, Rubbioli, Menin, Brandi, Auler, Rubbioli, Menin and Brandi2019), which means that current caves were already present in rock formations at least a few hundred thousand years ago.
Since the Lower Palaeocene (66 Mya) until today, many climate changes cycles (glacial and interglacial periods) occurred that initiated the genesis of vegetation adapted to the dry climate in South America (Cox et al. Reference Cox, Moore and Ladle2016), possibly influencing cave environments along these ranges, considering that most organic resources that supply caves come from the surrounding landscape (Kováč Reference Kováč, Moldovan, Kováč and Halse2018). These climate changes cycles resulted in dry forests (e.g., Chaco, Savanas, Cerrado and Caatinga) and humid forests (Amazon and Atlantic Rain Forest) (Ledo & Coli 2017, Olson et al. Reference Olson, Dinerstein, Wikramanayake, Burgess, Powell, Underwood, D’amico, Itoua, Strand, Morrison, Loucks, Allnutt, Ricketts, Kura, John, Lamoreux, Wettengel, Hedao and Kassem2001, Werneck et al. Reference Werneck, Costa, Colli, Prado and Sites2011). However, during the glaciation and interglaciation periods, several connections were established/lost between humid and dry forests (Cox et al. Reference Cox, Moore and Ladle2016). These events are widely known to have led to the isolation and connection of different taxonomic groups in different ecosystems (Sobral-Souza et al. Reference Sobral-Souza, Lima-Ribeiro and Solferini2015, Vitorino et al. Reference Vitorino, Lima-Ribeiro, Terribile and Collevatti2018, Vivo Reference Vivo1997, Werneck et al. Reference Werneck, Costa, Colli, Prado and Sites2011), including hypogean environments (Pérez-González et al. Reference Pérez-González, Ceccarelli, Monte, Proud, DaSilva and Bichuette2017, Polhemus & Ferreira Reference Polhemus and Ferreira2018).
Forests and caves can be environmental “refuges” for organisms that evolved or transited in many of these paleoenvironments (Bryson et al. Reference Bryson, Prendini, Savary and Pearman2014, Pointing et al. Reference Pointing, Bollard-Breen and Gillman2014). Consequently, the combined study of epigean (forests) and hypogean habitats (caves) may allow a better understanding of the biogeographic history of cave-dependent taxonomic groups (Pérez-González et al. Reference Pérez-González, Ceccarelli, Monte, Proud, DaSilva and Bichuette2017). This combined assessment allows the establishment of the relictual species and trace possible connections, routes and reservoir used by previous lineages in response to large-scale climate change, e.g., glaciations and interglaciations (Bryson et al. Reference Bryson, Prendini, Savary and Pearman2014, Pointing et al. Reference Pointing, Bollard-Breen and Gillman2014).
Cave crickets belong to the suborder Ensifera (Insecta: Orthoptera) and are omnivorous or scavengers (Deharveng & Bedos Reference Deharveng, Bedos, Moldovan, Kováč and Halse2018). In the Neotropical region, the most diverse family of cave crickets is Phalangopsidae (Ensifera: Grylloidea) (Deharveng & Bedos Reference Deharveng, Bedos, Moldovan, Kováč and Halse2018, Desutter-Grandcolas Reference Desutter-Grandcolas1995). These crickets are active at night while remaining sheltered during the day, refuging in hollow trees, crevices, burrows or under dead branches (Desutter-Grandcolas Reference Desutter-Grandcolas1993). Hence, their nocturnal habit favoured the use of caves as shelters, and a large number of species can be found in such subterranean environments, using caves as diary refuge (“cavicolous” sensu Desutter-Grandcolas Reference Desutter-Grandcolas1995) or living strictly inside the caves due to isolation processes (troglobitic sensu Desutter-Grandcolas Reference Desutter-Grandcolas1995).
In particular, the genus Endecous (Orthoptera: Grylloidea: Phalangopsidae) could be a good model group for large-scale studies as they present species occurring in both epigean and hypogean environments (Bolfarini & Bichuette Reference Bolfarini and Bichuette2015, Castro-Souza et al. Reference Castro-Souza, Zefa and Ferreira2020a, Reference Castro-Souza, Junta and Ferreira2020b, Souza-Dias et al. Reference Souza-Dias, Bolfarini, Nihei and Mello2014). In addition, resources derived from cave crickets, such as dung, bodies, carcases and eggs, can directly be consumed by several other cave invertebrate species, thus contributing to the structuring of subterranean communities (Lavoie et al. Reference Lavoie, Helf and Poulson2007, Mammola et al. Reference Mammola, Cardoso, Culver, Deharveng, Ferreira, Fišer, Galassi, Griebler, Halse, Humphreys, Isaia, Malard, Martinez, Moldovan, Niemiller, Pavlek, Reboleira, Souza-Silva, Teeling, Wynne and Zagmajster2019a, Taylor Reference Taylor and Gunn2003, Taylor et al. Reference Taylor, Krejca and Denight2005). This shows their biological relevance as a key group for ecological and evolutionary studies in caves (Bento et al. Reference Bento, Souza-Silva, Vasconcellos, Bellini, Prous and Ferreira2021, Fagan et al. Reference Fagan, Lutscher and Schneider2007, Lavoie et al. Reference Lavoie, Helf and Poulson2007). The maintenance of key groups in caves is essential for subterranean communities’ conservation and a better understanding of the temporal dynamics in these environments.
However, the true spatial distribution of Endecous (Wallacean shortfall) as well as many caves are unknown (Racovitzan shortfall) (see Ficetola et al. Reference Ficetola, Canedoli and Stoch2019, Hortal et al. Reference Hortal, de Bello, Diniz-Filho, Lewinsohn, Lobo and Ladle2015). Considering that current global warming affects in both epigean and hypogean habitats (Mammola et al. Reference Mammola, Piano, Cardoso, Vernon, Domínguez-Villar, Culver, Pipan and Isaia2019b), it is expected that Endecous crickets can present changes in their spatial distribution over time as adaptive response (see Bellard et al. Reference Bellard, Bertelsmeier, Leadley, Thuiller and Courchamp2012). Therefore, the use of species distribution models (SDMs) (Guisan & Thuiller Reference Guisan and Thuiller2005, Peterson et al. Reference Peterson, Soberón, Pearson, Anderson, Martínez-Meyer, Nakamura and Araújo2011) provides a better understanding of temporal dynamics (past, present and future) and detects historically stable areas (stability consensus), when different scenarios of these models overlap (Carnaval et al. Reference Carnaval, Hickerson, Haddad, Rodrigues and Moritz2009, Sobral-Souza et al. Reference Sobral-Souza, Vancine, Ribeiro and Lima-Ribeiro2018, Terribile et al. Reference Terribile, Lima-Ribeiro, Araujo, Bizao, Collevatt, Dobrovolski, Franco, Guilhaumon, Lima, Murakami and Nabout2012). Such models allow to infer the potential impacts of climate change on Endecous ecology and conservation in the face of its potential distribution.
Here, we aimed to predict the biogeographic history and future distribution pattern of the crickets Endecous in South America, as well as to understand the spatio-temporal rearrangements of the genus to detect possible refuges. We hypothesized that (i) in the past (LGM 21 ka) the potential distribution of the genus Endecous was geographically wider, highlighting potential links between cave environments as result of forest vegetation expansion (Sobral-Souza et al. Reference Sobral-Souza, Lima-Ribeiro and Solferini2015) and (ii) in the future, the potential distribution of the genus Endecous will be reduced and restrict to caves from South America, as a direct result of restriction of forest habitats.
Materials and methods
Dataset compilation for Endecous Saussure (1878) occurrences
Niche models are based on environmental attributes suitable for species occurrence (Smith et al. Reference Smith, Godsoe, Rodríguez-Sánchez, Wang and Warren2019). Since most of the species known for the genus Endecous are not formally described (Castro-Souza et al. Reference Castro-Souza, Zefa and Ferreira2020a), and some of them only have a single occurrence (Cigliano et al. Reference Cigliano, Braun, Eades and Otte2022), we considered here the genus taxonomic level, since the genus monophyly is well supported in the cladistic literature for Phalangopsidae (Souza-Dias et al. Reference Souza-Dias2015). Furthermore, most species in this genus exhibit a strong association with cave environments, unlike groups found in the same monophyletic clade of Endecous (genera Luzarida, Melanotes and Palpigera, sensu Souza-Dias (Reference Souza-Dias2015)), that have never been found inhabiting caves (hypogean environment), but leaf litter, tree trunks and holes or interstices up in tree (Cigliano et al. Reference Cigliano, Braun, Eades and Otte2022; Desutter-Grandcolas Reference Desutter-Grandcolas1995). In particular, Endecous species present apparently similar ecological niches in such subterranean habitats (e.g., similar dietary requirements, distribution inside caves), which justifies the use of the genus in such analysis.
Data occurrences of the genus Endecous were obtained from different sources. (i) Taxonomic Literature: references available in Orthoptera Species File Online (Cigliano et al. Reference Cigliano, Braun, Eades and Otte2022);9 (ii) Photographs that allowed identifying the presence of the genus in online platforms: Platform iNaturalist (research from family Phalangopsidae, genus Endecous; available at https://www.inaturalist.org/); Blog (available in http://www.blog.gpme.org.br/); Personal files of specialists in subterranean biology (Ferreira, R.L.; Souza-Silva, M.; Cardoso, R.C., Rabelo, L.R.); (iii) Bioespeleological Studies and Orthoptera Ecological Studies: papers, dissertations, thesis and academic abstracts; (iv) Expert Observation: mentioned in the literature of studies with Endecous species (e.g., Mello, F.A.G.; Zefa, E.; Souza-Dias P.G.B; Bolfarini M.P.; Acosta, R.C.).; (v) ISLA Collection: specimens cataloged in the “Collection of Subterranean Invertebrates of Lavras” (ISLA), Center of Studies on Subterranean Biology, Department of Ecology and Conservation, Federal University of Lavras, Brazil.; (vi) Technical Reports: studies required by the Brazilian legislation to carry out projects in areas with caves or to regulate cave visitation. In this case, some of these studies presented locations with photos of fauna that enabled the identification and confirmation of the genus on site.
A total of 516 occurrences were registered (Figure 1, Supplementary Table 1). The georeferencing of the occurrences was obtained with the geographic coordinate system WGS84 and clustered in two categories: (I) “Records” (data with exact coordinates) and (II) “Approximate record” (data lacking exact coordinates and based on descriptive reference, e.g., municipality, city, etc.). For the Approximate Record data, we obtained the geographic coordinate by analysing satellite images of habitat and forest patches nearest the centroid described in the bibliography. Since the model’s building was carried out with a 0.5º resolution grid cell, the approximate records do not jeopardize it. Data from sources whose taxonomic identification was not conclusive or confirmed were excluded. Then, with the georeferenced occurrences, we made a grid of cells with a resolution of 0.5° (∼55 × 55 km) in the South American region. We filtered only one point of occurrence within each cell, thus totalling 124 cells with Endecous records (Figure 1b). This procedure made it possible to reduce the sampling bias, as the occurrence data tend to be concentrated in more studied areas.
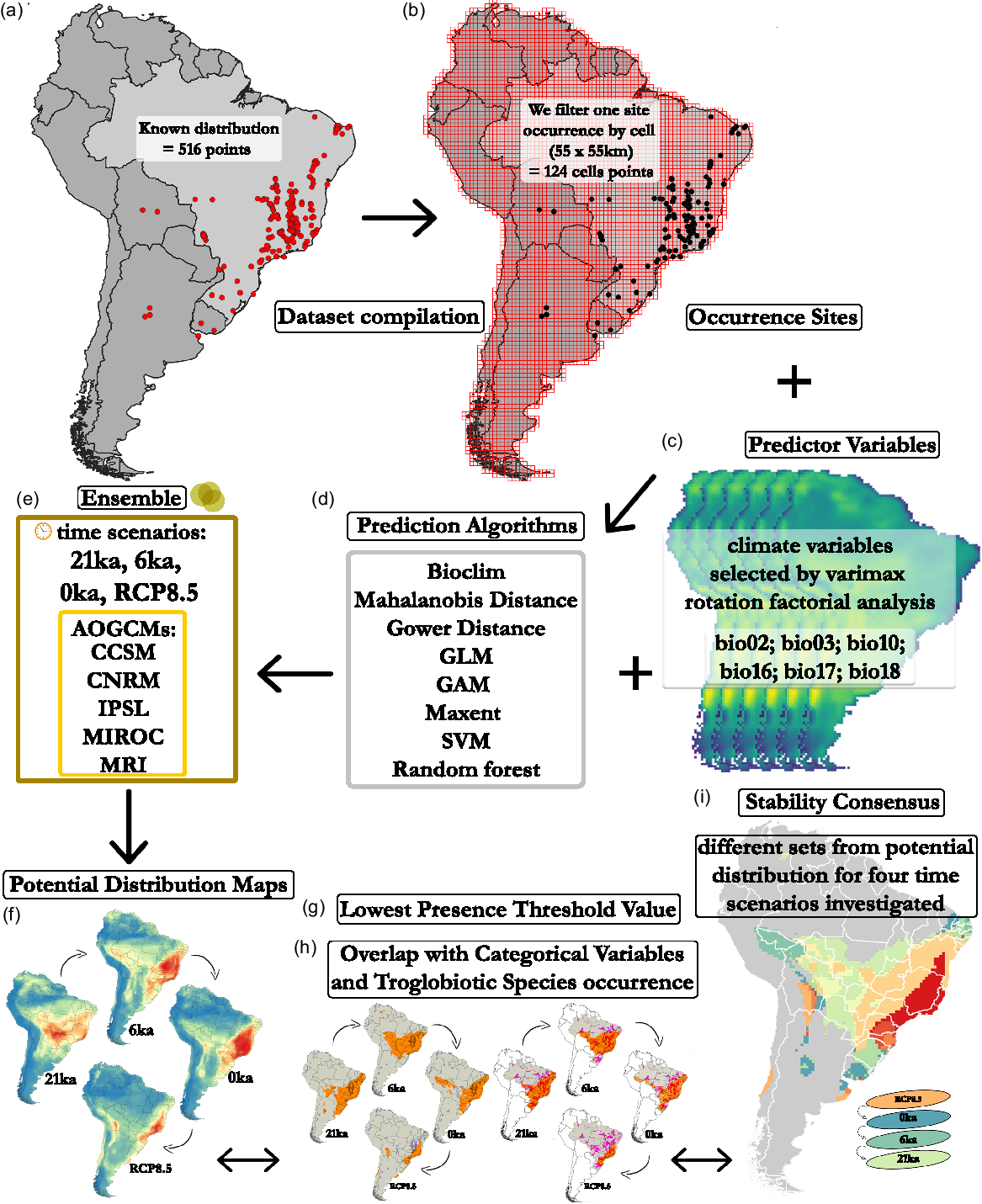
Figure 1. Outline summarizing our methodological and analytical steps to predict the potential distribution of the genus Endecous Saussure, 1878. The first step consisted in compiling as much data as known for the distribution of the genus (a). Next, we filter the occurrence data, leaving only one presence point per grid (b). The third step consists of associating the occurrence sites (b), predictor environmental variables (c), and algorithms (d), to building models. For this, we use the method of Ensemble (e), which consists of superimposing the models for each algorithm, inside each AOGCM, for each time scenario investigated (e). Then consensus maps are built for the genus (f). Next, we use the Lowest Predicted Value Threshold (lpt) to create binary presence maps, as of the lowest predicted value on environmental suitability according to the occurrence sites. Finally, binary maps overlapped with categorical variables and occurrence sites of troglobitic species. In addition, we also overlap the binary maps in the four-scenario investigated to check possible areas climatically stable of the niche potential of Endecous.
In order to determine the paleo and the current distribution, as well as the future genus distribution in South America, we used the bioclimatic quantitative data obtained from five Atmospheric-Ocean Global Circulation Models (AOGCMs) adapted from Lima-Ribeiro et al. (Reference Lima-Ribeiro, Varela, González-Hernández, de Oliveira, Diniz-Filho and Terribile2015): (1) CCSM4; (2) CNRM-CM5; (3) IPSL-CM5ALR; (4) MIROC-ESM; (5) MRI-CGCM3. Since these models represent the temporal dynamics and climate conditions of the planet, they enable us to simulate the past, present and future climate conditions. The use of multiple AOGCMs simulations is strongly suggested in SDMs studies (Zurrell et al. Reference Zurell, Franklin, König, Bouchet, Dormann, Elith, Fandos, Feng, Guillera-Arroita, Guisan and Lahoz-Monfort2020).
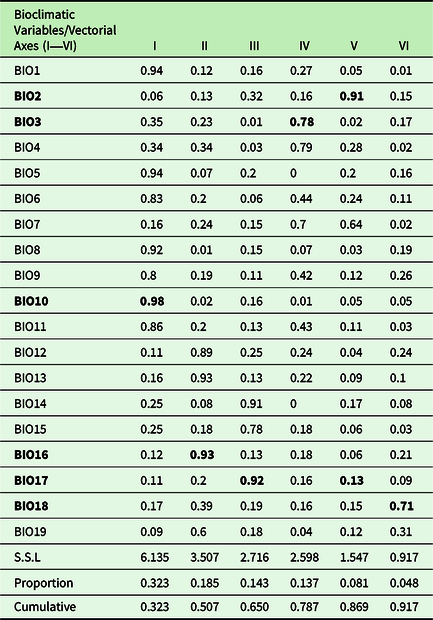
Since there might be distinct patterns presented by the AOGCMs, we used different climate projections (see Varela et al. Reference Varela, Lima-Ribeiro and Terribile2015). For each AOGCMs, six bioclimatic variables from the EcoClimate database (http://www.ecoclimate.org) were used: Bio02 = Mean Diurnal Temperature Range (mean of monthly (max temp – min temp)); Bio03 = Isothermality; Bio10 = Mean Temperature of Warmest Quarter; Bio16, Bio17, Bio18 (precipitation of Wettest, Driest and Warmest Quarter, respectively). These variables were selected from a total of 19 available (Lima-Ribeiro et al. Reference Lima-Ribeiro, Varela, González-Hernández, de Oliveira, Diniz-Filho and Terribile2015), through Varimax rotation factorial analysis (Fávero et al. Reference Fávero, Belfiore, Silva and Chan2009) (Figure 1c, Table 1). The six selected variables showed low correlation with each other and high representativeness in the explanation of orthogonal axes.
Table 1. Results of varimax rotation factorial analysis, used for the selection of more explanatory variables among the 19 bioclimatic variables (Nix Reference Nix and Longmore1986, Hijmans et al. Reference Hijmans, Cameron, Parra, Jones and Jarvis2005) for the construction of SDMs
Niche modelling of the genus Endecous Saussure 1878
Different mathematical algorithms that have been currently used for niche models building aimed to infer species spatial distribution patterns (Barry & Elith Reference Barry and Elith2006, Diniz-Filho et al. Reference Diniz-Filho, Bini, Rangel, Loyola, Hof, Nogués-Bravo and Araújo2009, Zurrell et al. Reference Zurell, Franklin, König, Bouchet, Dormann, Elith, Fandos, Feng, Guillera-Arroita, Guisan and Lahoz-Monfort2020). We used eight algorithms to test the potential spatial distribution of the genus Endecous in four temporal predicted scenarios: 21 ka (Last Glacial Maximum), 6 ka (mid-Holocene), 0 ka (1950–1999, current) and RCP 8.5 (mean of simulations for 2080–2100, with increasing CO2 emissions towards the end of the 21st century) (Figure 1d). Such algorithms can be categorized as: i—algorithms of presence: Bioclim (Nix Reference Nix and Longmore1986); Mahalanobis Distance (Farber & Kadmon Reference Farber and Kadmon2003) and Domain – Gower distance (Carpenter et al. Reference Carpenter, Gillison and Winter1993); ii— algorithms of presence/absence: General Linear Model (GLMz) (Guisan et al. Reference Guisan, Edwards and Hastie2002) and General Additive Model (GAM) (Guisan et al. Reference Guisan, Edwards and Hastie2002); iii—Machine Learning Algorithms: Support Vector Machines (SVM) (Tax & Duin Reference Tax and Duin2004), Maximum Entropy (Phillips & Dudik Reference Phillips and Dudik2008) and Random Forest (RDNFOR) (Guisan et al. Reference Guisan, Edwards and Hastie2002).
For each algorithm, 10 randomly repetitions were performed using the bootstrapping partition, with double partitioning for occurrence sites (70% training and 30% test), in order to evaluate the model predictive power (true skill statistic (TSS)) (Allouche et al. Reference Allouche, Tsoar and Kadmon2006). TSS values close to one (1) were classified as good, zero (0) values were classified as random and negative values (close to −1) were classified as poor models.
The ensemble method was used to concatenate all built models in a single map (Araújo & New Reference Araújo and New2007): first, the models obtained in each algorithm (10 replicates), then between the algorithms (8 algorithms), and finally the AOGCM (5AGCMs) (Figure 1e). A total of 400 models were built (10 models × 8 algorithms × 5 AOGCMS) for each temporal period (21 ka, 6 ka, 0 ka and 2100). Thus, each cell on the map has a prediction value ranging from 0 to 400, which, divided by 400, represents the prediction frequency of Endecous occurrence within the grid (Figure 1f; Supplementary Figure 1). Then, we used the Lowest Predicted Value Threshold (lpt) to create binary maps of presence (Pearson et al. Reference Pearson, Raxworthy, Nakamura and Peterson2007) (Figure 1g).
The temporal binary maps were compared with the South American karstic areas (shapefile available at: https://www.fos.auckland.ac.nz/our_research/karst/. Accessed March 21, 2021), and the records of troglobitic Endecous species (only occurring in caves) (Figure 1h). The records of cave-restricted species were not used for the niche models building since it was assumed they were not being influenced directly by external climatic variables used on the models, as the remaining species (which are troglophilic, thus, presenting external populations). Furthermore, we also contrasted the binary maps with the Cave Occurrence Areas in Brazil, data provided by the National Center for Cave Research and Conservation (CECAV) (shapefile available at: https://www.icmbio.gov.br/cecav/projetos-e-atividades/provincias-espeleologicas.html. Accessed March 21, 2021) (Figure 1h). Finally, we ensembled the temporal binary potential distribution maps to obtain consensus on areas of climatic stability within the possible potential niche of Endecous (representing potential refuges for the genus) (Figure 1i).
All analyses mentioned above were performed in R (R Development Core Team, 2021). Species distribution models were built using dismo package (Hijmans et al. Reference Hijmans, Phillips, Leathwick and Elith2022). Maps were made through QGIS 3.4 (Free 2021) and Inkscape (Inkscape Team 2004–2021) software.
Results
The niche models showed great adjustments, with TSS values greater than 0.5 for each algorithm analysed (Table 2). The six bioclimatic variables selected explained approximately 91% of the environmental variation in South America climate conditions.
Table 2. True Skill Statistic (TSS) values for each combination of algorithms and AOGCM for SDMs of the genus Endecous Saussure, 1878

The temporal predictions showed that Endecous had a wide spatial distribution 21 ka ago (LGM), extending along central and western South America, with additional small patches in northwestern South America, between Colombia and Venezuela. During the LGM, the Endecous distribution coincided with the main existing Brazilian karstic areas (e.g., Araras, Corumbá, Açungui, Bambuí and Una geological groups) and with two karstic areas of Bolivia and Paraguay (Figure 2, 21 ka).
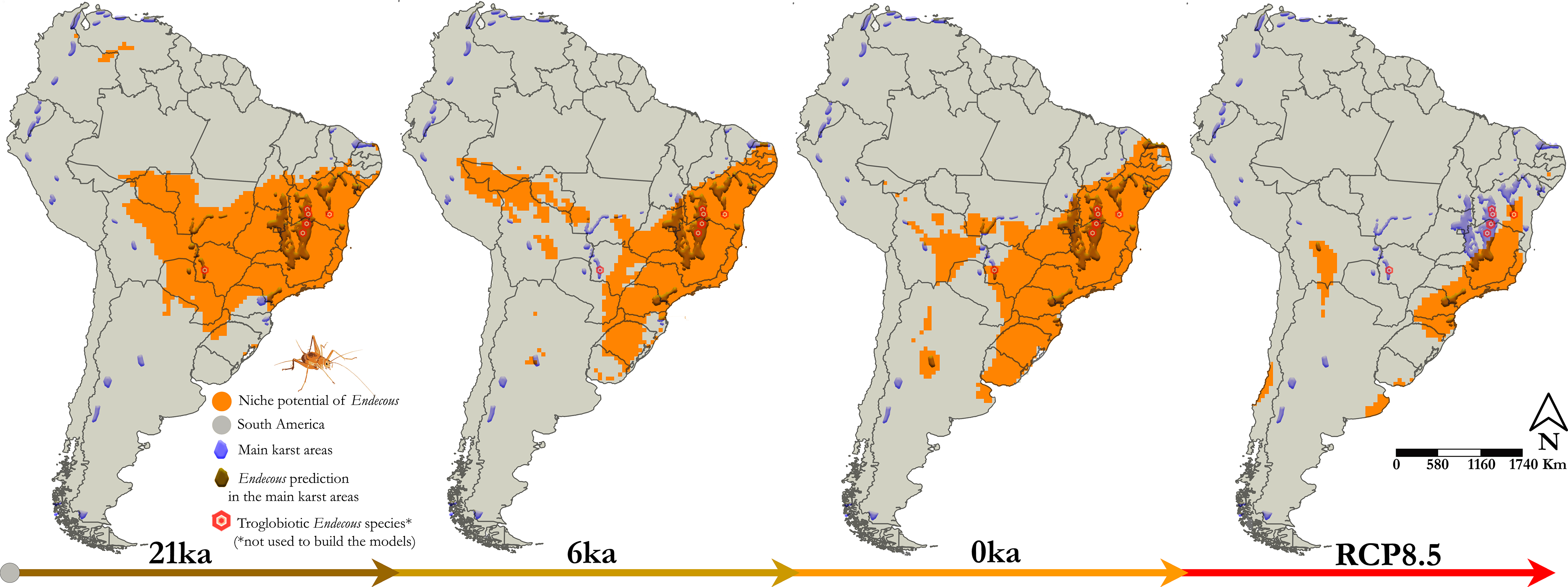
Figure 2. Potential distribution for genus Endecous Saussure, 1878 during Last Glacial Maximum (21 ka), Mid-Holocene (6 ka), Current (0 ka) and Future (RCP8.5, 2080–2100). The models were overlapping with the main karst areas in South America, since most of the genus is facultative in caves. The colouring of the arrows represents the temperature rise in each scenario (cold ⇨ hot).
The spatial paleodistribution was fragmented in the middle Holocene (6 ka) with occupancy losses in central South America, in the Araras and Corumbá carbonatic groups, respectively, located in the States of Mato Grosso and Mato Grosso do Sul (Brazil). However, a slight geographical expansion occurred in southern and northeastern Brazil, including part of the Apodi carbonatic group. Distribution patches may also be verified bordering Peru and Bolivia, southern Argentina and Andean regions during the mid-holocene (Figure 2, 6 ka).
The current Endecous potential spatial distribution is broader than the known occurrence data (Figure 1a), incorporating areas of central and northeastern South America. The predictions showed a distribution pattern similar to 6 ka ago and presented spatial losses and rearrangements along the distribution patches, mainly in the Andean region. In Brazil, there is an area gain in the central region, covering again some karst regions that had been lost in the middle Holocene (Araras and Corumbá groups). Furthermore, the distribution of Endecous has expanded towards northeastern and southern Brazil, with southward expansions to other countries, such as Uruguay and part of eastern Argentina (Figure 2, 0 ka).
The spatial predictions to future climate scenarios (RCP8.5, 2080–2100) showed a potential decrease in spatial occupancy and occurrence of Endecous in karstic areas of northeastern and southeastern South America. Its distribution will mainly occur in a continuous area in eastern and southeastern Brazil. Smaller occupancy patches are expected in southern Uruguay and eastern Argentina and throughout the central and southern Andean region, comprising the borders with Bolivia, Argentina and Chile, respectively. Such distributional retractions exclude possible occurrences of the genus in all karstic areas of Argentina and the entire territory of Paraguay (Figure 2, RCP8.5).
Regarding the analysis of Endecous distribution in cave occurrence areas in Brazil, the models showed that 21,000 years ago all Endecous populations were established throughout all biomes and occupied large karstic areas, likely inhabiting caves that already exist in these regions (Figure 3, 21 ka). However, it also showed that there was a reduction in Endecous distribution in central Brazil approximately 6,000 years ago, and the same pattern has been observed for the current scenario (Figure 3, 6 ka and 0 ka). The predictions for future distribution patterns (2080–2100) show that a rearrangement could occur with an intensive decrease in the genus distribution in areas of cave occurrence in central, southeastern and northeastern Brazil (Figure 3, RCP8.5).

Figure 3. Potential distribution for genus Endecous Saussure, 1878 during Last Glacial Maximum (21 ka), Mid-Holocene (6 ka), Current (0 ka) and Future (RCP8.5, 2080–2100). The models were overlapping the main areas with the occurrence of caves from Brazil, since most known data in this study come from these environments. The colouring of the arrows represents the temperature rise in each scenario (cold ⇨ hot).
The models revealed possible refuges for Endecous in the four scenarios analyzed (21 ka ∩ 6 ka ∩ 0 ka ∩ RCP8.5), including epigean and hypogean environments in Brazil (Figure 4a and b – karst areas in red color). On the epigean regions, its distribution remained stable mainly in Atlantic rainforest regions, although it also comprised two smaller regions of the Caatinga (semi-arid) and Cerrado (savanna) biomes (Figure 4). In the hypogean environment, the most stable regions (21 ka ∩ 6 ka ∩ 0 ka ∩ RCP8.5) comprised part of the Açungui group and the southernmost Bambuí and Una groups. In addition, in at least one of the temporal scenarios, the distribution encompasses karstic areas of South America (Figure 4b – karstic areas in orange).
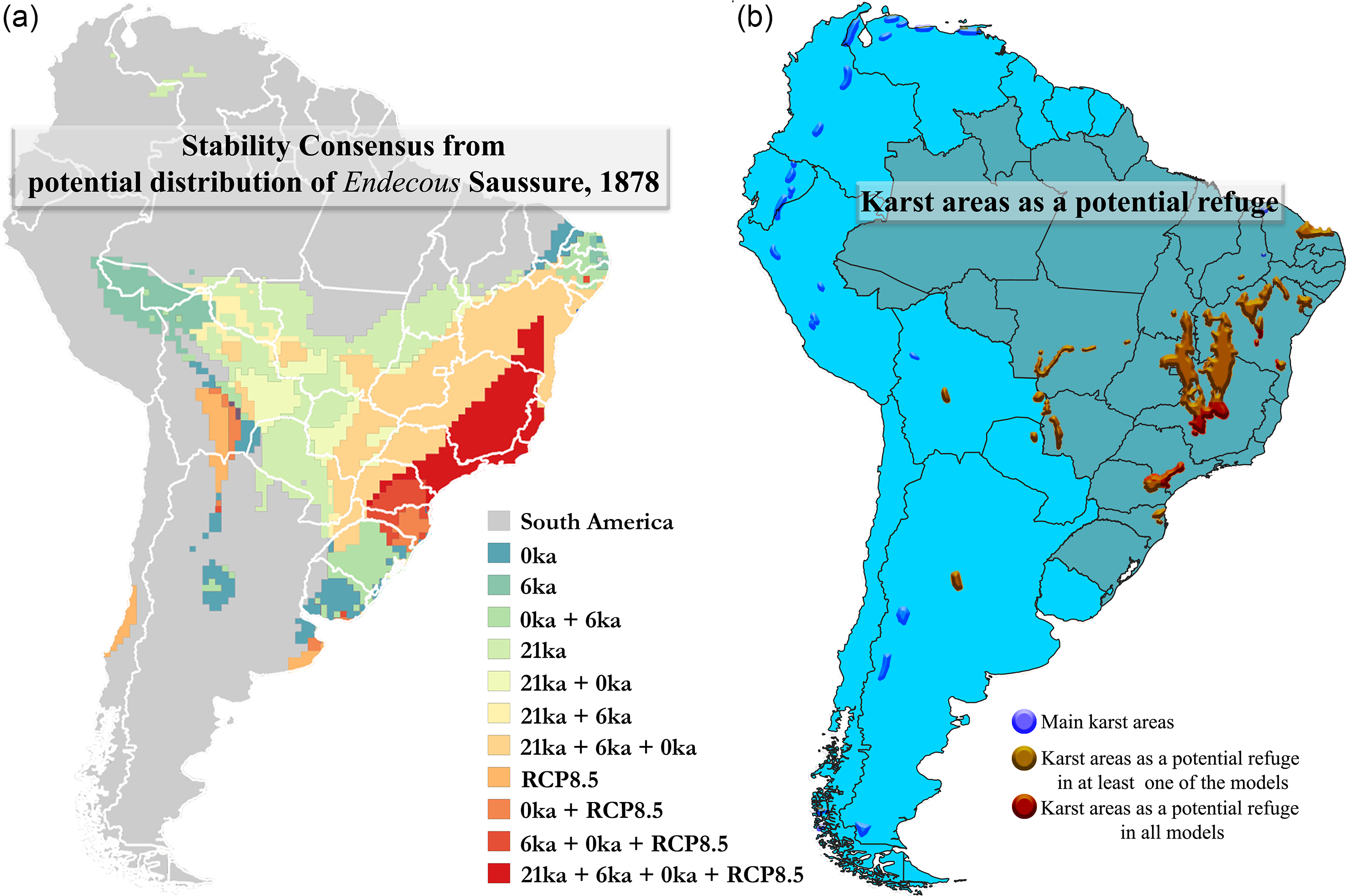
Figure 4. (a) Stability Consensus comparing different combinations between the four scenarios the potential distribution for genus Endecous Saussure, 1878. Legend: Last Glacial Maximum (21 ka), Mid-Holocene (6 ka), Current (0 ka) and Future (RCP8.5, 2080–2100); (b) Karst areas as a potential refuge from Species distribution models (SDMs) of the Endecous Saussure, 1878.
Discussion
Our findings suggest that the Endecous spatial distribution occupied a broad region in central and western South America, covering and linking groups of areas where caves were present (Figures 2 and 3, scenarios 21 ka and 6 ka). The current distribution scenario (0 ka) was similar to 6 ka, showing rearrangements and expansions to the northeast and south of South America (Figures 2 and 3, scenario 0 ka). For the future scenario, the predictions are worrying, as there is a retraction of almost two-thirds of the potential areas for the genus occurrence, isolating several regions with caves that were previously connected, which may indicate a reduction of suitable climatic areas and the isolation of Endecous in epigean and hypogean environments (Figures 2 and 3, scenario RCP8.5, 2080–2100).
By overlaying all models, it is possible to detect potential refuges for the genus in eastern and southeastern South America, mainly in the Atlantic Rainforests (Figures 2 and 3, overlapping scenarios 21 ka, 6 ka, 0 ka, RCP8.5). Furthermore, considering the current impacts on epigean environments, the conservation of karst landscapes can represent a vital action to preserve several Endecous lineages.
The Atlantic Rainforests as a source and temporal refuge of Endecous crickets
The current distribution and paleodistribution of Endecous encompass the late Pleistocene expansion and retraction cycles of humid forests in South America (see Ledo & Colli Reference Ledo and Colli2017, Sobral-Souza et al. Reference Sobral-Souza, Lima-Ribeiro and Solferini2015). Furthermore, their distribution in areas currently known as dry forest diagonal (Dinerstein et al. Reference Dinerstein, Olson, Joshi, Vynne, Burgess, Wikramanayake, Hahn, Palminteri, Hedao, Noss, Hansen, Locke, Ellis, Jones, Barber, Hayes, Kormos, Martin, Crist, Sechrest, Price, Baillie, Weeden, Suckling, Davis, Sizer, Moore, Thau, Birch, Potapov, Turubanova, Tyukavina, de Souza, Pintea, Brito, Llewellyn, Miller, Patzelt, Ghazanfar, Timberlake, Klöser, Shennan-Farpón, Kindt, Barnekow Lillesø, van Breugel, Graudal, Voge, Al-Shammari and Saleem2017, Olson et al. Reference Olson, Dinerstein, Wikramanayake, Burgess, Powell, Underwood, D’amico, Itoua, Strand, Morrison, Loucks, Allnutt, Ricketts, Kura, John, Lamoreux, Wettengel, Hedao and Kassem2001), similarly to 6,000 and 21,000 years ago (e.g., Werneck et al. Reference Werneck, Costa, Colli, Prado and Sites2011, Reference Werneck, Nogueira, Colli, Sites and Costa2012), indicates that these areas could have been transitional and expansion zones, where the Endecous could have colonized and established in caves. In addition, the presence of small refuges, such as hypogean habitats and/or vestiges of humid forests and riparian forests, or even certain preference for more shaded phytophysiognomies (e.g., Cerrado and Riparian Forests) in arid locations, may explain the occurrence of Endecous along dry forests, and in turn the constant presence of Endecous inhabiting caves in arid biomes (Castro-Souza et al. Reference Castro-Souza, Zefa and Ferreira2017, Souza-Dias et al. Reference Souza-Dias, Bolfarini, Nihei and Mello2014, Zefa et al. Reference Zefa, Redu, Costa, Gottschalk, Padilha, Silva and Martins2014).
The abovementioned explanations corroborate many studies indicating that the dry diagonal was a permeable barrier (due to several persistent patches of wet vegetation) that split the South American humid forests into the Amazon and Atlantic Forest (Johnson et al. Reference Johnson, Saraiva and Coelho1999, Redford & Fonseca Reference Redford and da Fonseca1986, Weisenberg & Mori Reference Weisenberg and Mori2020). The current distribution of many taxonomic groups, such as arachnids (Peres et al. Reference Peres, Silva and Solferini2017), small mammals (Costa Reference Costa2003, Redford & Fonseca Reference Redford and da Fonseca1986), birds (Silva Reference Silva1996, Willis Reference Willis1992), reptiles (Weisenberg & Mori Reference Weisenberg and Mori2020), anurans (Vasconcelos & Doro Reference Vasconcelos and Doro2016), butterflies (Cabette et al. Reference Cabette, Souza, Shimano and Juen2017, Matos-Maraví et al. Reference Matos-Maraví, Wahlberg, Freitas, Devries, Antonelli and Penz2021) and crickets (De Campos et al. Reference De Campos, Souza-Dias, Desutter-Grandcolas and Nihei2021) inside remnant humid forests along the dry diagonal is explained by this past separation of humid forests.
The spatial rearrangements (21 ka, 6 ka, 0 ka and RCP8.5) indicated that the most stable occurrence areas of Endecous are associated with the Atlantic Forest. This highlights moisture as a threshold condition for these crickets, since they depend on substrate moisture for oviposition (De Farias-Martins et al. Reference De Farias-Martins, Sperber, Albeny-Simões, Breaux, Fianco and Szinwelski2017). Considering that caves frequently present high moisture content and climatic stability (Mejía-Ortíz et al. Reference Mejía-Ortíz, Christman, Pipan and Culver2021, Moldovan et al. Reference Moldovan, Kováč and Halse2018, Sánchez-Fernández et al. Reference Sánchez-Fernández, Rizzo, Bourdeau, Cieslak, Comas, Faille, Fresneda, Lleopart, Millán, Montes and Pallares2018), the frequent observations of the genus associated with climatically stable regions within caves also coincide to what is exposed by the models. Furthermore, cave crickets may have sensitivity to desiccation (Lavoie et al. Reference Lavoie, Helf and Poulson2007) what favours their association to hypogean environments.
It is plausible to assume that humid forests have played an important role as population reservoirs for the genus Endecous. Furthermore, since the genus was continuously distributed over a vast geographic region, connections between caves were established, along with colonization events in such environments, supporting our first hypothesis.
Analysis in different temporal scenarios (21 ka, 6 ka, 0 ka and RCP8.5) based on the current Endecous distribution showed that these crickets almost did not reach the north of the Amazon biome. The same pattern was observed for the sympatric genus Eidmanacris Chopard, 1956 (Castro-Souza et al. Reference Castro-Souza, Junta and Ferreira2020b), a group that is also dependent on remnants of humid forests and cave habitats (De Campos et al. Reference De Campos, Souza-Dias, Desutter-Grandcolas and Nihei2021). Although these two genera can co-occur inside caves, there is spatial segregation between their populations, with Endecous inhabiting deeper regions and Eidmanacris occurring near cave entrances, which would avoid competitive exclusion (Castro-Souza et al. Reference Castro-Souza, Junta and Ferreira2020b, Paixão et al. Reference Paixão, Ferreira and Paixão2017).
In the Amazon biome, other genera of phalangopsids have been registered inside caves, as Uvaroviella Chopard, 1923 and Phalangopsis Serville, 1831. Additionally, studies in 844 caves in this region have never reported Endecous or Eidmanacris (supplementary material, Jaffe et al. Reference Jaffe, Prous, Zampaulo, Giannini, Imperatriz-Fonseca, Maurity, Oliveira, Brandi and Siqueira2016). Accordingly, it is plausible to infer that its dispersion is limited to the dry diagonal and Atlantic rainforests during the glacial and interglacial cycles. It is also possible that if there had been populations of Endecous in the Amazon biome in the past, interspecific competition might have occurred between Endecous and other crickets of similar habitat preference, as the genus Phalangopsis. This could have led to the exclusion of Endecous from the Amazon biome.
Another factor to be considered is that the Endecous species cannot fly because they are micropterous or apterous, which limits their dispersion through migration corridors with suitable habitats (e.g., forest habitats or caves, according to what we know). This also helps to explain the current and paleodistribution of the genus along the forests and caves inside of the Cerrado biome, since there is a greater floristic and climatic similarity between the Cerrado and the Atlantic Forest, than the Cerrado and the Amazon forest (Méio et al. Reference Méio, Freitas, Jatobá, Silva, Ribeiro and Henriques2003, Nimer Reference Nimer1989).
The future predictions (2080–2100) showed that the potential distribution of Endecous crickets would become restricted on epigean habitats, with its potential isolation in caves in South America, corroborating our second hypothesis. This habitat reduction or restriction can be intensified because favourable epigean habitats, such as the Cerrado and Atlantic Rainforests, have been strongly threatened by agricultural expansion, urbanization and mining (Dinerstein et al. Reference Dinerstein, Olson, Joshi, Vynne, Burgess, Wikramanayake, Hahn, Palminteri, Hedao, Noss, Hansen, Locke, Ellis, Jones, Barber, Hayes, Kormos, Martin, Crist, Sechrest, Price, Baillie, Weeden, Suckling, Davis, Sizer, Moore, Thau, Birch, Potapov, Turubanova, Tyukavina, de Souza, Pintea, Brito, Llewellyn, Miller, Patzelt, Ghazanfar, Timberlake, Klöser, Shennan-Farpón, Kindt, Barnekow Lillesø, van Breugel, Graudal, Voge, Al-Shammari and Saleem2017, MapBiomas Project 2021, Mittermeier et al. Reference Mittermeier, Gil, Hoffmann, Pilgrim, Brooks, Mittermeier, Lamoreux and Da Fonseca2004, Myers et al. Reference Myers, Mittermeier, Mittermeier, Da Fonseca and Kent2000, Souza et al. Reference Souza, Shimbo, Rosa, Parente, Alencar, Rudorff, Hasenack, Matsumoto, Ferreira, Souza-Filho and de Oliveira2020, Sugai et al. Reference Sugai, Ochoa-Quintero, Costa-Pereira and Roque2015).
The continued deforestation of the Atlantic Forest has intensified in Brazil (2018–2019), especially in State of Minas Gerais (INPE 2021, SOS Mata Atlântica 2021). The Brazilian political–environmental scenario often brings bills (e.g., PL 3729/04, PL 191/20 (Brasil 2021a, 2021b)) seeking to use regions currently protected and conserved by law. This certainly represents a constant threat, which makes the effects of future climate change even more severe for the ecology of these crickets and associated subterranean biota.
Finally, although our models have indicated the genus is more intimately associated with the Atlantic Forest than with the dry diagonal, it is not possible to address any origin for the genus without an updated and broadly taxon-inclusive phylogeny. Furthermore, many of our records came from caves (hypogean environments) since it is easier to find and sample Endecous species in such habitats. Thus, due to the extensive sampling gaps in surface environments, further samplings along the dry diagonal and the Amazon forest are recommended, as well as a taxonomic review of the genus, accompanied by molecular phylogeny. Only then, it will be possible to understand more precisely the biogeographic pathways used by the different species of this genus.
Caves as potential refuges for the species conservation in the future
The Atlantic Rainforest and subterranean environments are the main refuges for the crickets Endecous. Furthermore, due to the fact that the hypogean environments are more climatically stable than epigean (Badino Reference Badino2010, Brookfield et al. Reference Brookfield, Macpherson and Covington2016, Mammola et al. Reference Mammola, Piano, Cardoso, Vernon, Domínguez-Villar, Culver, Pipan and Isaia2019b), many Endecous populations distributed in caves were less vulnerable to climatic oscillations that occurred at the surface in the past and are likely to occur in the future (2080–2100). Hence, considering that caves are important refuges, and that such environments can become, in some areas, the only suitable habitat for species in the face of global warming, cave preservation is crucial for the maintenance of many populations of different species.
Brazilian’s legislation on the protection of the spelaeological heritage provides for a categorization of caves into levels of relevance (maximum, high, medium or low), being the caves of ‘maximum relevance’ the only ones fully protected (Decree No. 6640 of November 7, 2008 and Normative Instruction No. 01/2017, Ganem Reference Ganem2009). However, this legislation is currently under strong political debate, due to the proposal of a retrograde decree (nº. 10.935/2022) which allows the destruction of any cavities, even those considered as presenting maximum relevance (Ferreira et al. Reference Ferreira, Bernard, da Cruz Júnior, Piló, Calux, Souza-Silva, Barlow, Pompeu, Cardoso, Mammola, García, Jeffery, Shear, Medellín, Wynne, Borges, Kamimura, Pipan, Hajna and Sendra2022). Furthermore, most Endecous species are ‘troglophiles’ (facultative in caves), and only few species are restricted to cave habitats (troglobitic). Therefore, this may be a setback for Endecous conservation, since their presence would not be enough to classify a cave as ‘maximum relevance’, reinforcing that such habitats may be crucial for many species in the future.
Although we did not use records of troglobitic Endecous in our dataset, when superimposing such occurrences with the models built, it is evident that the ancestors of these species colonized the caves in the past, since the potential paleodistribution of the genus was wide and encompassed regions where such species currently occur. Thus, it is important to emphasize that facultative cave ancestors have great potential to become cave specialists in the future (Chapman Reference Chapman1982), with Endecous being a living example, given that it is one of the only two genera of crickets in Brazil with troglobitic species (Bolfarini & Bichuette Reference Bolfarini and Bichuette2015, Castro-Souza et al. Reference Castro-Souza, Zefa and Ferreira2020a, Souza-Dias et al. Reference Souza-Dias, Bolfarini, Nihei and Mello2014). Furthermore, geographically isolated refuges may have contributed to the increase in speciation within caves (e.g., Castro-Souza et al. Reference Castro-Souza, Zefa and Ferreira2020a), which increases the taxonomic complexity of the genus (Castro-Souza, R.A & Ferreira, R.L. unpubl. data from analysis of DNA barcoding).
In addition, a phalangopsid (Luzarinae) found in an amber from the early Miocene (around 20 million years from the Dominican Republic (Heads Reference Heads2010), demonstrates the ancient history of occurrence of this group in the Americas and allows us to assume that many ancestors of phalangopsid species could already be colonizing existing caves due to their nocturnal and foraging lifestyle. In particular, many South American cave phalangopsidae (e.g. Adelosgryllus, Endecous, Phalangopsis and Strinatia) (Campos et al. Reference Campos, Souza-Dias and Nihei2017, Castro-Souza et al. Reference Castro-Souza, Junta and Ferreira2020b, Cigliano et al. Reference Cigliano, Braun, Eades and Otte2022, Junta et al. Reference Junta, Castro-Souza and Ferreira2020, Merlo et al. Reference Merlo, Castro-Souza, Junta and Ferreira2022, Mesa et al. Reference Mesa, Garcia and Zefa1999) reveal pre-adaptations reported for other cave crickets (Desutter-Grandcolas Reference Desutter-Grandcolas1993; Desutter-Grandcolas Reference Desutter-Grandcolas1997; Heads Reference Heads2010), such as eye size reduced, loss of ocelli, absence of auditory tympana and body discoloration. However, to confirm that the traits are pre-adaptations, it is necessary to evaluate them together with the habitat from a phylogenetic perspective (Desutter-Grandcolas Reference Desutter-Grandcolas1997). Thus, the past investigated in our study (the last 21 ka) likely represents just the “tip of the iceberg” in the biogeographic history of South American cave crickets, as uncountable events of colonization, speciation and extinction may have occurred in different caves along the continent, which highlights the need for more studies of evolution and biogeography of this group.
Caves are more than a refuge to the Endecous species. They are natural laboratories that play an important role on the biodiversity conservation and the understanding of evolutive processes which this crickets has been undergoing. These subterranean environments are also a source of important biological information regarding the evolutive lineages of this group. Furthermore, the study of key species, hereby represented by the genus Endecous, will certainly aid on the conservation of subterranean ecosystems (Wynne et al. Reference Wynne, Howarth, Mammola, Ferreira, Cardoso, Lorenzo, Galassi, Medellin, Miller, Sánchez-Fernández and Bichuette2021). This information may surrogate more well-defined conservation actions, which should not only aim at troglobite species, but also at some key facultative cave-dwelling groups. Although some organisms inhabiting caves are not restricted to these environments, they may be undergoing speciation processes and may also be key species to the subterranean environment. The data presented in this study broadens the knowledge of the crickets Endecous and highlights the importance of cave conservation for better biodiversity conservation policies.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467422000529
Acknowledgments
The authors thank the team of the Center for Studies on Subterranean Biology (CEBS) of the Lavras Federal University (UFLA) for support in field trips. We are especially grateful to Ecoclimate database, institutions and platforms that encourage and/or allow to support the data for such a study: CAPES, CECAV; CNPq, FAPEMIG, FAPESP, OSF and VALE S.A. The authors are grateful to Dra. Erika Taylor for help in English translating this study. RACS is grateful to Dr. Neucir Szinwelski, Ms. Riuler Acosta, Ms. Nicolas Bosco and Ms. Victor Prasniewski for encouraging and teaching the Orthopterology. RLF is grateful to the CNPq (grant nº 308334/2018-3).
Author contributions
Rodrigo Antônio Castro-Souza: Conceptualization; data curation; formal analysis; methodology; writing. Thadeu Sobral-Souza: Conceptualization, formal analysis, methodology, review and editing the manuscript. Lucas Mendes Rabelo: Conceptualization, data curation, review and editing the manuscript. Edison Zefa: writing; review and editing the manuscript. Rodrigo Lopes Ferreira: Conceptualization, data curation, methodology, supervision, review and editing the manuscript. All authors read and approved the final manuscript.
Conflict of interest
All authors declare that they have no conflicts of interest.
Funding
RACS received a scholarship by VALE S.A.; RLF is funded by CNPq (grant nº 308334/2018-3).