Introduction
Sarpa salpa, commonly called Salema, is a Sparidae that lives in shallow seas where seagrass beds like Posidonia oceanica and Cymodocea nodosa are abundant. The species prefers temperate and tropical areas; it is widely distributed in the eastern Atlantic, western Indian Ocean, some areas of the Black Sea, and the Mediterranean Sea (Criscoli et al., Reference Criscoli, Colloca, Carpentieri, Belluscio and Giandomenico2006). This fish is a demersal herbivore (Verlaque, Reference Verlaque1990) that feeds on algae, diatoms, and macrophytes (Havelange et al., Reference Havelange, Lepoint, Dauby and Bouquegneau1997). However, this fish is rarely eaten worldwide, with the exception of France and Tunisia. Indeed, it is very appreciated in the south of Tunisia, especially during its reproduction season when it stops feeding to spawn (Anato and Ktari, Reference Anato and Ktari1983). Because of those reasons, we chose to discriminate the S. salpa stock at the two most important consumer sites in Tunisia: Djerba and Kerkennah, using otolith morphology and microchemistry analysis.
Otoliths, mineralized concretions found in the inner ears of teleost fish, are composed of three pairs positioned in endolymph-filled cavities on either side of the brain. The Sagittae is the largest; it is distinguished by two sides (concave and convex), two projections (rostrum and antirostrum), and a furrow (Sulcus acusticus) (Lecomte-Finiger, Reference Lecomte-Finiger1999). The balance and hearing senses depend on these calcareous concretions for correct functionality (Popper et al., Reference Popper, Ramcharitar and Campana2005).
Otoliths are produced by adding calcium carbonate (CaCO3), minor elements (Na, Sr, K, S, Cl, P), traces (Mg, Si, Zn, B, Fe, Hg, Mn, Ba, Cu, Al, Br, Li), and infratrace (Pb, As, Se, Ag, Co, Cd, U, Cs) to a protein matrix (otolin) (Campana, Reference Campana1999). They record events in fish life history due to their metabolically inert composition (Campana and Neilson, Reference Campana and Neilson1985). Thus, they are used for many biological investigations, such as species identification (Rousset, Reference Rousset1983; Maisey, Reference Maisey1987); palaeontological studies (Nolf, Reference Nolf1993; Steurbaut and Nolf, Reference Steurbaut and Nolf1998); growth analysis; age determination; and fish mortality analysis (Cardinale et al., Reference Cardinale, Doering-Arjes, Kastowsky and Mosegaard2004).
In recent years, otoliths have become widely used in fish stock analyses. So, many researchers in Tunisia have used otolith morphology to discriminate stocks of various species (Messaoud et al., Reference Messaoud, Bouriga, Daly Yahia, Boumaiza, Faure, Quignard and Trabelsi2011; Trojette et al., Reference Trojette, Ben Faleh, Fatnassi, Marsaoui, Mahouachi, Chalah, Quignard and Trabelsi2015; Rebaya et al., Reference Rebaya, Ben Faleh, Allaya, Khedher, Marsaoui, Chalah, Quignard and Trabelsi2016, Reference Rebaya, Ben Faleh, Allaya, Khedher, Trojette, Marsaoui, Fatnassi, Chalah, Quignard and Trabelsi2017; Mejri et al., Reference Mejri, Trojette, Allaya, Ben Faleh, Jmil Chalah, Quignard and Trabelsi2018a, Reference Mejri, Trojette, Allaya, Ben Faleh, Jmil, Chalah, Quignard and Trabelsi2018b; Ben Mohamed et al., Reference Ben Mohamed, Mejri, Ben Faleh, Allaya, Jmil, Rebaya, Chalah, Quignard and Trabelsi2019; Jmil et al., Reference Jmil, Ben Faleh, Rebaya, Allaya, Ben Mohamed, Trojette, Chalah, Quignard and Trabelsi2019; Bakkari et al., Reference Bakkari, Mejri, Ben Mohamed, Chalah, Quignard and Trabelsi2020; Ben Labidi et al., Reference Ben Labidi, Mejri, Shahin, Quignard, Trabelsi and Ben Faleh2020a, Reference Ben Labidi, Mejri, Shahin, Quignard, Trabelsi and Ben Faleh2020b; Bouriga et al., Reference Bouriga, Mejri, Dekhil, Bejaoui, Quignard and Trabelsi2021; Khedher et al., Reference Khedher, Mejri, Shahin, Quiganrd, Trabelsi and Ben Faleh2021).
Although several scientists around the world have used microchemical analysis to discriminate fish stocks (Gordon et al., Reference Gordon, Swan, Geffen and Morales-Nin2001; Swan et al., Reference Swan, Gordon and Shimmield2003; Veinott and Porter, Reference Veinott and Porter2005; Miyana et al., Reference Miyana, Khana, Patel, Khana and Ansar2016), no scientific research has used this method for fish stock evaluation. The fish stock evaluation allows the determination of the population's degree of isolation from other populations of the same species.
However, scientific research based on the fluctuating asymmetry of otolith shape has been used for stock discrimination in Tunisia (Mejri et al., Reference Mejri, Trojette, Jmil, Ben Faleh, Chalah, Quignard and Trabelsi2020, Reference Mejri, Bakkari, Tazarki, Mili, Chalh, Shahin, Quignard, Trabelsi and Ben Faleh2022). Fluctuating asymmetry can be defined as deviations from a known ratio of morphological structure. As a particular type of phenotypic variability, the fluctuating asymmetry level is an indicator of optimal conditions for development and genetic coadaptation. Thus, fluctuating asymmetry acts as a measure of developmental state and stability in population biology (Zakharov and Trofimov, Reference Zakharov and Trofimov2022). Thus, otolith fluctuating asymmetry is both appealing and simple to use, and it has been found to vary between populations, hence having the potential to be useful for fisheries evaluation (Diaz-Gil et al., Reference Diaz-Gil, Palmer, Catalan, Alos, Fuiman, Garcia, Del-Mar-Gil, Grau, Kang, Maneja, Mohan, Morro, Schaffler, Buttay, Riera-Batle, Tolosa and Morales-Nin2015).
Fish stock is the basic unit used in the assessment of fisheries states and the application of management policies for the implementation of sustainable development (Tanner et al., Reference Tanner, Reis-Santos and Cabral2015). In fact, in this study, we performed three different otolithometric analyses to discriminate the S. salpa stock captured from Djerba and Kerkennah, Tunisian islands. So, firstly, we used elliptical Fourier descriptors (EFD) to analyse the otoliths’ morphology. Secondly, the otoliths’ biometry was examined in terms of length, width, air, perimeter, and weight. Finally, a microchemical analysis was performed for K, Cs, and Pb. Those heavy metals have been chosen for the microchemical analysis given their importance in the Gulf of Gabes ecological analyses and especially given that most of the scientific research in Tunisia is interested in these elements (Chouba et al., Reference Chouba, Mastouri and El Abed1996; Mkawar et al., Reference Mkawar, Azri, Kamoun and Montacer2007; Rabaoui et al., Reference Rabaoui, Balti, El Zrelli and Tlig-Zouari2013).
The main goals were to discriminate the S. salpa stocks on the islands of Djerba and Kerkennah, to determine fluctuating asymmetry in both populations, to check sexual stock dimorphism in each population, and to test the feasibility of otolith morphological and microchemical analyses as a new tool for stock evaluation.
Materials and methods
Sample collection
120 adult specimens of S. salpa were collected from the two islands (Djerba and Kerkennah) (Figure 1) by artisanal fishing (gill nets) in October 2021. Therefore, 60 fish (30 males and 30 females) were studied from each station. In fact, using an ichthyometer and a balance, the total length (LT) and total weight (WT) of each specimen were recorded to the nearest mm and 0.01 g, respectively. Longmore et al. (Reference Longmore, Fogarty, Neat, Brophy, Milton and Mariani2010) report that for otolith morphological and microchemical analysis, it is necessary to study mature individuals with similar size and weight ranges to avoid confounding effects of ontogenesis on otolith chemistry and shape. Thus, our samples were selected based on their maturity and similar size. The variance comparison test of total length (LT) and weight was used to check the sample homogeneity. However, this homogeneity test is parametric, so the Shapiro–Wilks test for normality verification was first carried out for the lengths and the total weights.
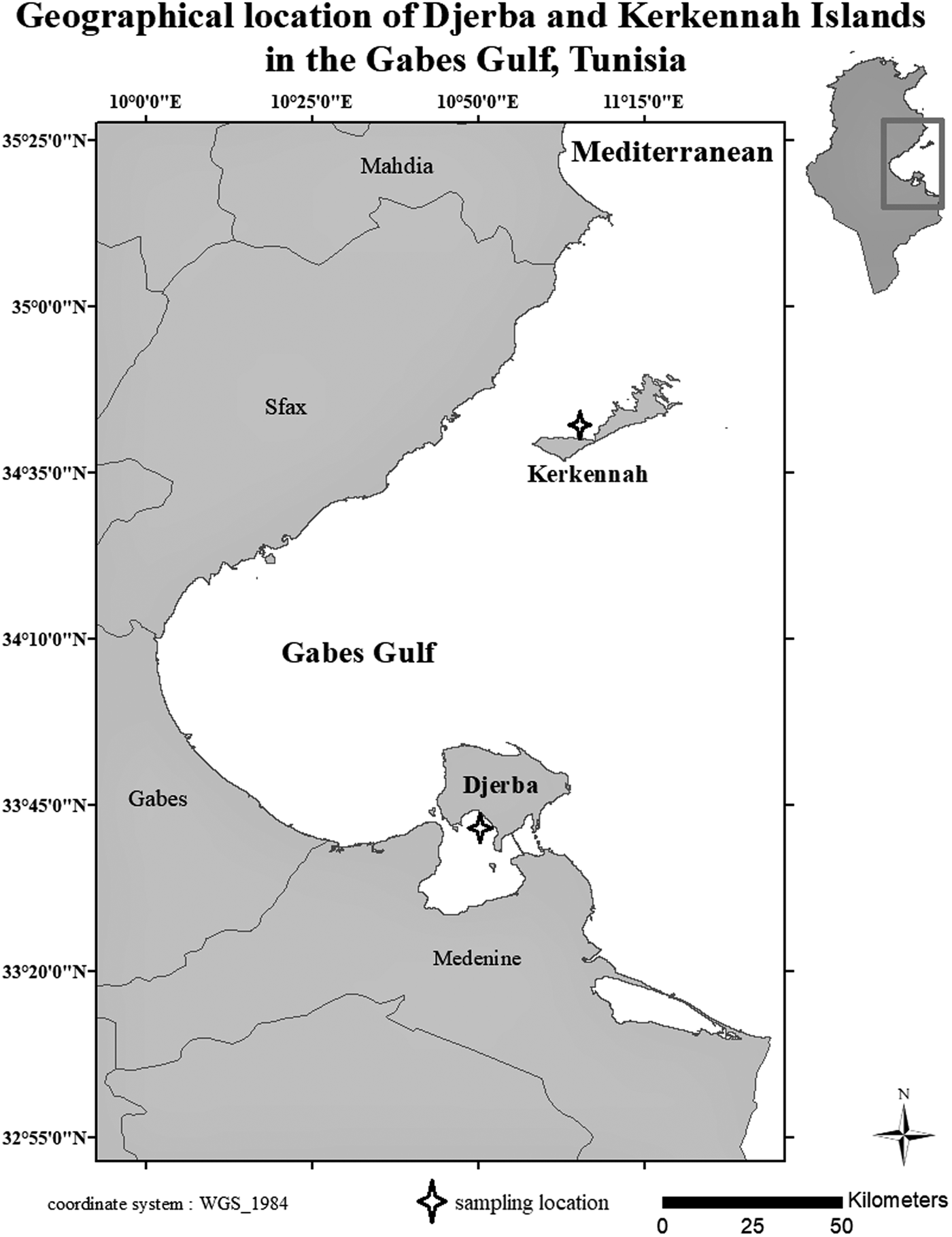
Figure 1. Sarpa salpa sampling sites on Djerba and Kerkennah islands, Tunisia.
Otolith extraction
Firstly, a small chisel was used to make a horizontal cut on the upper bone of the fish head. Then, Sagittae pairs were extracted with forceps. They are then cleaned with distilled water, dried, and finally stored in Eppendorf tubes.
Otolith morphometric analysis
Both right and left sagittal otoliths were placed on a microscope slide, and each sagittae was photographed using a digital camera (Canon IXUS 185, 20 MP). Otoliths were positioned with the sulcus facing up and the concave side above. Otolith photos previously converted into BMP format were processed with SHAPE software Ver. 1.3 (Iwata and Ukai, Reference Iwata and Ukai2002). Firstly, a chain code employing a binary contour projection was used to create the otolith shape. Secondly, EFD were created from the chain code. The EFDs were normalized, and twenty harmonics were extracted for each otolith. For each harmonic, four coefficients (a, b, c, and d) were distributed. Thus, for each otolith, a total of 80 coefficients was obtained; therefore, the principal component analysis was carried out to summarize the information of the EFD in four principal coefficients.
The otolith morphology measurements for length (Lo), width (Wo), area (Ao), perimeter (Po), and weight (Mo) were determined using ImageJ (Figure 2). To avoid the statistical analyses being impacted by the change in pixel count, we used the identical otolith image in both the Shape and the ImageJ software. Additionally, right and left otolith weights were measured in grams using a balance precision of 0.01 mg. Using Shapiro–Wilks’ test, the normal distribution of otolith morphological variables was tested. If the variables do not follow the normal distribution, a Box–Cox transformation (Box and Cox, Reference Box and Cox1964) is performed. Then, the comparison test between the variances of the two samples was carried out. Therefore, the MANOVA was performed on the otolith morphological variables (the four elliptical Fourier coefficients, the length, the width, the perimeter, the area, and the weight). Finally, the discriminant factor analysis (DFA) was applied to the otolith morphological variables and expressed with the tests of lambda Wilks and p-values of Fisher distances. These statistical tests were first used to compare the two populations, then to verify the fluctuating asymmetry of each population by comparing the morphological variables of the right and left otoliths of each population. The sexual dimorphism of the population was finally verified by comparing statistical variables between males and females at each site.
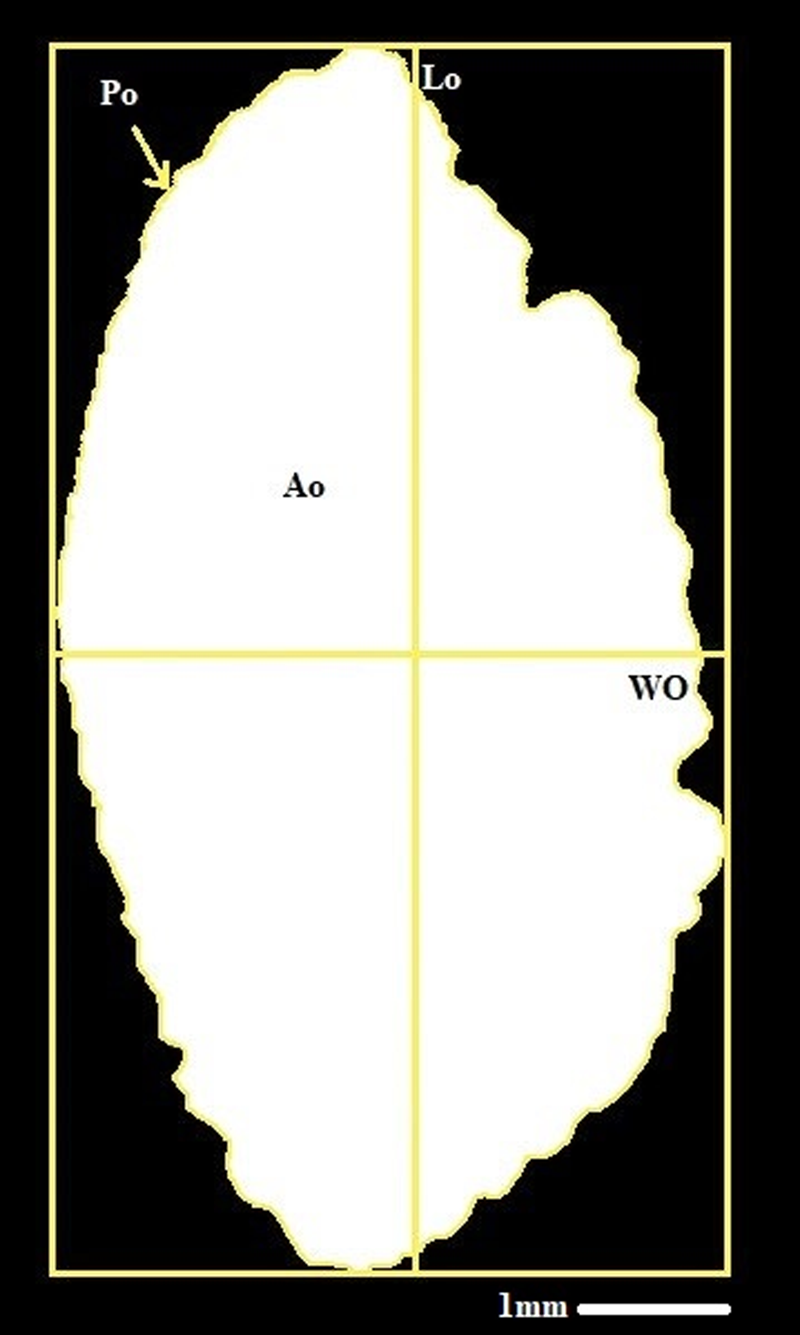
Figure 2. Left otolith of Sarpa-Salpa and its biometric parameter, scale bars: 1mm.
Otolith microchemical analysis
All otoliths were crushed to a fine powder in a mortar that had been cleaned with acetone to prevent contamination. Then 1 g of otolith powder was mineralized by adding 8 mL of HNO3 solution (65%) and 2 mL of H2O2 solution. The resulting mixture was heated on a hot block at 125 °C ± 5 °C before being dissolved in 50 ml of ultrapure water (Milli Q). The results of this mineralization were then analysed by Flame Atomic Absorption Spectrometry (FAAS), the Analytik Jena (Novaa350) model. Acetylene is injected into the spectrometer's flame at a rate of 70 L/h with a 6 mm burner height. The wavelengths used to detect K, Cs, and Pb are 766.5 nm, 852.1 nm, 228.8 nm, and 283.3 nm, respectively. Therefore, we obtained the content of K, Cs, and Pb for 1 g of otolith, and since we measured the weight of the otolith on a precision balance, we calculated the content of each mineral element (K, Cs, and Pb). We then used a discriminant factor analysis (DFA) on all of the values to compare the two populations using microchemical analysis.
Results
Sample homogeneity
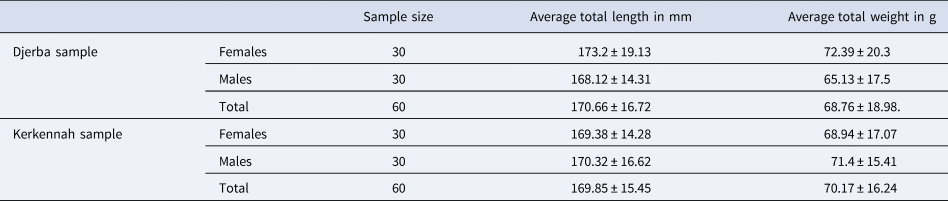
The S. salpa sampled at Djerba had an average total length of 170.66 ± 16.72 mm and a mean total weight of 68.76 ± 18.98g. However, the S. salpa collected in Kerkennah had an average total length of 169.85 ± 15.45 mm and an average total weight of 70.17 ± 16.24 g (Table 1).
Table 1. Biometric measurements of Sarpa salpa
The Shapiro–Wilks test showed that the sample followed the normal distribution with a p-value 0.78 (>0.05). The homogeneity test of variance comparison performed on the fish's total length (LT) and total weight (WT) proves the absence of a significant difference between samples (LT p-value = 0.22; PT p-value = 0.54). This allows us to eliminate the effect of size variation on the otolith's shape.
Comparison between the two populations
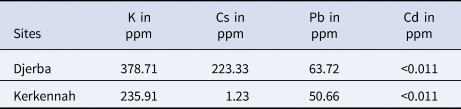
The MANOVA test performed on the otolith morphological variables showed a p-value <0.0001. This result was confirmed by Wilk's lambda test of DFA, which also gave a p-value of less than 0.0001 (Table 2). Otolith microchemical analysis shows that the contents of K, Cs, and Pb are higher in Djerba (K = 378.71 ppm, Cs = 223.33 ppm, and Pb = 63.72 ppm) than in Kerkennah (K = 235.91 ppm, Cs = 1.23 ppm, and Pb = 50.66 ppm) (Table 3). The Wilk's lambda test of otolith microchemical analyses gave a p-value < 0.0001 (Table 2). This means that the otolith microchemical analysis confirms the morphological analysis. Both microchemical and morphological otolith analyses prove that Djerba and Kerkennah stocks are significantly different.
Table 2. Wilks’ Lambda test for otoliths morphology, and microchemistry of Sarpa salpa sampled from Djerba and Kerkennah, Tunisia; executed on left and right otolith
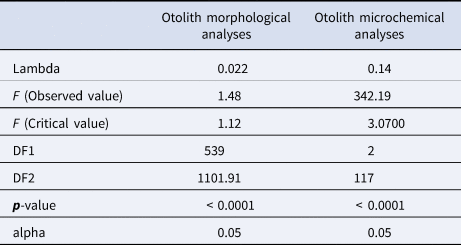
Table 3. Heavy metals analysis (K, Cs, and Pb) in the otoliths of Sarpa salpa sampled from the Tunisian islands of Djerba and Kerkennah
Fluctuating asymmetry and sexual dimorphism in the Djerba population
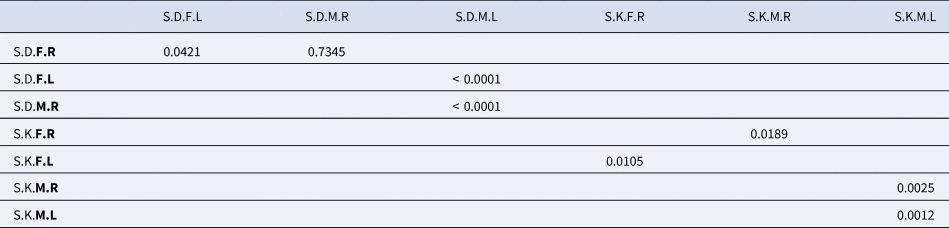
The Wilks lambda test performed on S. salpa sampled in Djerba revealed that both fluctuating asymmetry and sexual stock dimorphism are very significant (p-value <0.0001) (Table 2). The p-values for Fisher distances show that males have a higher fluctuating asymmetry (p-value <0.0001) than females (p-value = 0.0421). Furthermore, the p-value test for Fisher distances indicated that sexual dimorphism (p-value <0.0001) between left otoliths is more pronounced than that between right otoliths (p-value = 0.7345) (Table 4).
Table 4. P-value of the Fisher distances test for sexual dimorphism and fluctuating asymmetry in males and females of Sarpa salpa from Djerba and Kerkennah, Tunisia
Fluctuating asymmetry and sexual dimorphism in the Kerkennah population
Otolith morphology analysis showed that the fluctuating asymmetry and sexual stock dimorphism of the Kerkennah population are very important (Wilks’ Lambda p-value < 0.0001) (Table 2). However, males have a greater fluctuating asymmetry (p-value for the fisher distance = 0.0025) than females (p-value for the fisher distance = 0.0105). Furthermore, the Fisher distance p-value test for sexual dimorphism evaluation revealed a greater degree of significance between left otoliths (p-value = 0.0012) than between right otoliths (p-value = 0.0189) (Table 4).
Discussion
The results of otolith morphological and microchemical analysis showed a highly significant difference between the populations of Djerba and Kerkennah. The otolith fluctuating asymmetry was significant in both the populations of Djerba and Kerkennah, with a higher value in males than in females.
In the current study, the MANOVA results obtained from otolith morphological analysis for S. salpa demonstrate a significant difference between Djerba and Kerkennah. This finding is similar to those of Trojette et al. (Reference Trojette, Ben Faleh, Fatnassi, Marsaoui, Mahouachi, Chalah, Quignard and Trabelsi2015), who discovered significant variations in Diplodus annularis populations between Djerba and Kerkennah. The results can be explained by physicochemical differences between Djerba and Kerkennah, such as temperature (Cardinale et al., Reference Cardinale, Doering-Arjes, Kastowsky and Mosegaard2004), which is higher in Djerba (from 15 °C to 27 °C) than in Kerkennah (14 °C to 24.5 °C), according to the National Institute of Meteorology of Tunisia.
Many authors indicate that otolith formation is influenced by a synergistic action of various factors (Vignon, Reference Vignon2012), including environmental conditions (Vignon and Morat, Reference Vignon and Morat2010), food resources (Gagliano and Mccormic, Reference Gagliano and Mccormic2004; Mille et al., Reference Mille, Mahe, Cachera, Villanueva, De-Pontual and Ernande2016; Ben Labidi et al., Reference Ben Labidi, Mejri, Shahin, Quignard, Trabelsi and Ben Faleh2020a; Khedher et al., Reference Khedher, Mejri, Shahin, Quiganrd, Trabelsi and Ben Faleh2021; and Mejri et al., Reference Mejri, Trojette, Jmil, Ben Faleh, Chalah, Quignard and Trabelsi2020), and genetic characteristics (Vignon and Morat, Reference Vignon and Morat2010).
A significant difference between the stock of males and females was found for both the Djerba and Kerkennah populations. According to Cardinale et al. (Reference Cardinale, Doering-Arjes, Kastowsky and Mosegaard2004), sexual dimorphism has a considerable impact on the morphology of the otolith. The sexual dimorphism found in otoliths, according to Bakkari et al. (Reference Bakkari, Mejri, Ben Mohamed, Chalah, Quignard and Trabelsi2020), can be explained by genetic or environmental stress. Panfili et al. (Reference Panfili, Durand, Diop, Gourène and Simier2005) also explain sexual dimorphism as a fluctuation of factors associated to growth, fertility, or even survival. Shuster (Reference Shuster2009), on the other hand, defines otolith sexual dimorphism as a unique sound reception ability used to seek mating partners throughout the reproductive phase.
Both the Djerba and Kerkennah groups have considerable fluctuating asymmetry in otolith shape. Many authors have linked this imbalance to environmental stresses (Somarkis et al., Reference Somarkis, Kostikas and Tsimenid1997; Panfili et al., Reference Panfili, Durand, Diop, Gourène and Simier2005; Diaz-Gil et al., Reference Diaz-Gil, Palmer, Catalan, Alos, Fuiman, Garcia, Del-Mar-Gil, Grau, Kang, Maneja, Mohan, Morro, Schaffler, Buttay, Riera-Batle, Tolosa and Morales-Nin2015). Pollution, according to some authors, is the most important element influencing fluctuating asymmetry (Elsdon et al., Reference Elsdon, Wells, Campana, Gillanders, Jones, Limburg, Secor, Simon, Thorrold and Walther2008; Munday et al., Reference Munday, Hernaman, Dixson and Thorrold2011; Perry et al., Reference Perry, Redman, Widman, Meseck, King and Pereira2015; Kontas et al., Reference Kontas, Bostanci, Yedier, Kurucu and Polat2018). According to Wang (Reference Wang2002), heavy metals have a direct effect on otolith development. According to Rabaoui et al. (Reference Rabaoui, Balti, El Zrelli and Tlig-Zouari2013), the heavy metal content in the Gabes Gulf is extremely high.
Otolith microchemical analysis of S. salpa sampled from Djerba and Kerkennah confirms the presence of a significant heavy metal concentration of K, Cs, and Pb in the otolith composition, with different quantities within the two sites. This result confirms and explains the significant difference between the morphological analyses. However, the chemical composition of the otolith is strongly connected to the chemical composition of the environment (Campana and Neilson, Reference Campana and Neilson1985); therefore, we may deduce that the chemical properties of the Djerba and Kerkennah habitats differ.
Overall, through this investigation, we were able to distinguish between two distinct stocks of S. salpa. The otolith morphology and microchemistry of the S. salpa Djerba differ significantly from those of the Kerkennah. The difference can be explained by variations in environmental conditions and pollution stress, as seen by the high concentrations of heavy metals on both islands. Sexual dimorphism was seen in both the Djerba and Kerkennah populations, which can be linked to genetic and environmental stress. The study offers new perspectives for investigating the ecological consequences of the high content of heavy metals in these two environments as well as the impact of human consumption of S. salpa collected from Djerba and Kerkennah. It also provides unique data on the S. salpa population in Kerkennah and Djerba, as well as the use of otolith microchemistry and morphology as a potential method for fish stock distinction.
Data availability statement
Data that support the findings of this study are available from the corresponding author, [Meriam Ben Ghorbel], upon request.
Authors’ contributions
Meriam Ben Ghorbel carried out the sampling of S. salpa from Djerba and Kerkennah; she has done the otolith morphology, microchemical analysis, and statistical analysis. Also the article writing. Marwa Mejri corrects the article's redaction. Houeto Madel Floriane Adjibayo showed the working method of morphology analysis. Abdellah Chalh: advice for biostatistics. Jean-Pierre Quignard advises on otolith manipulations. Monia Trabelsi chose the subject of the research and coordinated between the researchers. Nawzet Bouriga coordinates the overall work.
Competing interests
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.








