Introduction
Foraminifera are unicellular organisms that are found in a wide variety of benthic and planktonic marine environments (Murray, Reference Murray1973) as well as in fresh water, and they play an important role in carbon cycling (Gooday et al., Reference Gooday, Levin, Linke, Heeger, Rowe and Pariente1992; Fontanier et al., Reference Fontanier, Mackensen, Jorissen, Anschutz, Licari and Griveaud2006). Multi-chambered and single-chambered, hard-shelled foraminifera have been well documented all over the world as recent and fossilized specimens (Loeblich & Tappan, Reference Loeblich and Tappan1988). Soft-walled, single-chambered foraminifera, or monothalamids, are not so well known because they are delicate, they do not fossilize well and have fewer distinguishing morphological characteristics. Early pioneering studies have discovered numerous new species of monothalamids that live in a wide range of marine locations, including the North and South Atlantic and Pacific Oceans, Arctic and Antarctic Oceans by the Challenger expeditions (Brady, Reference Brady1879, Reference Brady1881, Reference Brady1884; Jones, Reference Jones1994) and in the Atlantic Ocean (Claparède & Lachmann., Reference Claparède and Lachmann1859; Gruber, Reference Gruber1884; Rhumbler, Reference Rhumbler1904, Reference Rhumbler1935), Gullmar Fjord, Sweden, and Skagerrak Strait, Denmark (Goës, Reference Goës1894; Höglund, Reference Höglund1947; Nyholm, Reference Nyholm1955), Indian Ocean (Jones & Brady, Reference Jones and Brady1866; Heron-Allen & Earland, Reference Heron-Allen and Earland1914) and North Sea (Heron-Allen & Earland, Reference Heron-Allen and Earland1917). These monothalamids nevertheless represented only a minority of taxa of the foraminifera known at the time (Loeblich & Tappan, Reference Loeblich and Tappan1988).
It was not until the 21st century that more extensive worldwide surveys were made of benthic monothalamids, in marine settings covering intertidal zones down to abyssal depths of 4000 m. As well as providing descriptions of novel species and genera many of these studies have also included molecular phylogenetic data that have provided important information on the evolutionary history of monothalamid foraminifera. Examples of where the more recent surveys have been conducted include the Adriatic Sea (Sabbatini et al., Reference Sabbatini, Bonatto, Gooday, Morigi, Pancotti, Pucci and Negri2010, Reference Sabbatini, Bonatto, Bianchelli, Pusceddu, Danovaro and Negri2012, Reference Sabbatini, Nardelli, Morigi and Negri2013), Antarctica and the Southern Ocean (Gooday et al., Reference Gooday, Bowser and Bernhard1996; Pawlowski et al., Reference Pawlowski, Fahrni, Brykczynska, Habura and Bowser2002a; Majewski, Reference Majewski2005; Pawlowski et al., Reference Pawlowski, Fahrni, Guiard, Conlan, Hardecker, Habura and Bowser2005, Reference Pawlowski, Majewski, Longet, Guiard, Cedhagen, Gooday, Korsun, Habura and Bowser2008; Majewski et al., Reference Majewski, Lecroq, Sinniger and Pawlowski2007; Sinniger et al., Reference Sinniger, Lecroq, Majewski and Pawlowski2008; Cedhagen et al., Reference Cedhagen, Gooday and Pawlowski2009; Pawlowski & Majewski, Reference Pawlowski and Majewski2011), Black Sea (Gooday et al., Reference Gooday, Anikeeva and Sergeeva2006, Reference Gooday, Anikeeva and Pawlowski2010; Anikeeva et al., Reference Anikeeva, Sergeeva and Gooday2013; Anikeeva & Gooday, Reference Anikeeva and Gooday2016), Eastern Pacific coast of the USA (Bernhard et al., Reference Bernhard, Habura and Bowser2006), Hamble Estuary, England (Larkin & Gooday, Reference Larkin and Gooday2004), coast of Iceland (Voltski & Pawlowski, Reference Voltski and Pawlowski2015), North-east Atlantic (Gooday, Reference Gooday1986, Reference Gooday2002; Morigi et al., Reference Morigi, Sabbatini, Vitale, Pancotti, Gooday A, Duineveld, De Stigter, Danovaro and Negri2012), Svalbard fjords (Gooday et al., Reference Gooday, Bowser, Cedhagen, Cornelius, Hald and Korsun2005; Majewski et al., Reference Majewski, Pawłowski and Zajaczkowski2005; Sabbatini et al., Reference Sabbatini, Morigi, Negri and Gooday2007), Beagle Channel, South America (Gschwend et al., Reference Gschwend, Majda, Majewski and Pawlowski2016) and the Western Atlantic coast of the USA (Habura et al., Reference Habura, Goldstein, Broderick and Bowser2008; Altin et al., Reference Altin, Habura and Goldstein2009; Goldstein et al., Reference Goldstein, Habura, Richardson and Bowser2010; Altin-Ballero et al., Reference Altin-Ballero, Habura and Goldstein2013). Many new monothalamid lineages are currently also being discovered using environmental DNA (eDNA) for species which so far have no morphological counterparts (Habura et al., Reference Habura, Pawlowski, Hanes and Bowser2004, Reference Habura, Goldstein, Broderick and Bowser2008; Pawlowski et al., Reference Pawlowski, Fontaine, da Silva and Guiard2011, Reference Pawlowski, Esling, Lejzerowicz, Cedhagen and Wilding2014). There is no doubt nowadays that monothalamid foraminifera represent a more significant component of the marine benthos than was previously thought.
Intertidal monothalamids have so far been under-represented in the recent literature, possibly because they are quite hard to find in this more challenging environment for the foraminifera. If it is known where they are to be located, intertidal monothalamids have the advantage of being readily accessible for repeated sampling for combined, morphological, behavioural and molecular analyses. Previous studies in north-west Scotland have only covered deep-water and mainly hard-shelled taxa (Heron-Allen & Earland, Reference Heron-Allen and Earland1916; Edwards, Reference Edwards, Banner and Lord1982; Wilding, Reference Wilding2002; Murray, Reference Murray2003). Two studies have included some molecular characterization of monothalamids found in samples from Dunstaffnage Bay in north-west Scotland, but morphological data for them were lacking (Pawlowski & Holzmann, Reference Pawlowski and Holzmann2008; Pawlowski et al., Reference Pawlowski, Majewski, Longet, Guiard, Cedhagen, Gooday, Korsun, Habura and Bowser2008).
The aim of this study was to report on the wide diversity of novel morphological varieties of monothalamid foraminifera to be found in intertidal zones in the Lorn area of north-west Scotland, and which were particularly abundant in estuarine mudflats near to the mouths of two of the major sea lochs in the Lorn area, Loch Creran and Loch Etive.
Materials and methods
Sample collection and processing
From June 2020 to November 2021, samples of surface sediment, seaweed, pebbles and shell debris were collected regularly at low tide (0.4–1.1 m height above chart datum) from estuaries and beaches at several relatively unpolluted sites in the Lorn area of north-west Scotland (Table 1, Figure 1). These sites typically included either clean, undisturbed rockpools or surfaces made up of a mixture of firm mud and sand and dotted with abundant worm casts. Samples of the surface sediment of estuarine mud were skimmed off using a cheese slicer and put into 100–500 ml cylindrical plastic containers with airtight lids and filled with seawater. Pieces of seaweed or holdfasts of seaweed, shells and pebbles were put into pots with seawater without the sediment. In the laboratory the sample pots were settled next to a north-facing window in an unheated room and the lids removed.

Fig. 1. Location of sampling sites (1–9) at intertidal zones in the Lorn area of north-west Scotland (inset).
Table 1. Intertidal locations sampled for monothalamids

On the same day, samples of seaweed, stones or shells containing attached monothalamids were transferred to a Petri dish so that examination of fresh contents could be undertaken under a dissection microscope. Example specimens were photographed in situ under the dissecting microscope and then carefully detached from their substrates by nudging them sideways with a pair of watchmaker's forceps. They were then transferred to a well slide with artist's brushes for further examination under a high-powered microscope.
On the day of collection, the contents of the pots containing beach sediment were stirred and the sediment was left overnight to settle to allow the foraminifera to migrate to the surface (Goldstein et al., Reference Goldstein, Habura, Richardson and Bowser2010). If necessary, the sediment was sieved beforehand with a coarse 1 mm mesh to remove large debris, but sieving using a finer mesh was not needed and helped to avoid the loss of the smaller specimens. The next day, the top layer of the sediment was skimmed off with a turkey baster and placed into a Petri dish for examination under a dissection microscope. As soon as a monothalamid cell was spotted in the sediment, a pair of fine artist brushes were used to transfer it gently to a well slide containing the ambient solution. After 4–5 monothalamids had been placed in a well slide, a coverslip was applied. The foraminifera that were alive would almost immediately begin to extend their pseudopodia and they remained in good condition for up to 2 hours at least. The specimens were studied and photographed under both the dissection microscope and high-powered microscope.
One of the species of interest normally lay concealed within a cyst made up of sediment, such as a mud ball. The mud balls were extracted from the sediment by tipping most of the sediment out of a Petri dish, leaving the mud balls adherent to the bottom of the dish. Dissection of the cyst or mud ball would sometimes reveal the presence of a resident monothalamid, which was then moved to a well slide using the artist brushes.
Some species harbour magnetic particles and these monothalamids could be harvested in large numbers using a neodymium-alloy magnet. To do this, the magnet was pressed against and moved about underneath a Petri dish full of sediment, and with the magnet still in place, the sediment was gently rinsed off with seawater to reveal the attached magnetic organisms. The magnet was then removed and the specimens were treated as described above. To determine whether any other species that had been initially collected with the brush method also contained magnetic particles, the magnet was placed under the well slide containing the specimens, and those with magnetic particles would follow the movement of the magnet under the slide.
A coverslip-capture method was used for smaller specimens or those difficult to see in the sediment. Once the larger specimens had been extracted from a sample of sediment lying in a Petri dish, excess seawater was removed from the surface of the sediment with a Pasteur pipette and coverslips were rested lightly on top of the sediment and left there for a few hours to overnight. Each coverslip was then carefully removed with a pair of forceps, tipped slightly so that any adherent debris slid off, and was then inverted over a drop of seawater on a microscope slide for further observation.
All the forms are illustrated in their natural colour, as staining with dye was not necessary for this investigation. Low power photographs were taken of the foraminifera under a Trinocular Nikon SMZ-10 dissection scope with reflected lighting using a Nikon D3100 SLR camera connected to a large monitor via an HDMI cable. High magnification photographs were taken with the same camera set-up attached to a trinocular Nikon Optiphot equipped with bright-field, dark-field and phase contrast optics. Differential interference-like effects were obtained with oblique lighting, obtained by slightly shifting the position of the bright-field condenser aperture that is mounted on a turret assembly. Photographs and movies of live specimens captured under the dissection scope were correlated with those taken under the Optiphot microscope. Afterwards, the coverslips were removed from some of the preparations which were then preserved in 70% isopropyl alcohol, cleared in glycerol and re-mounted under coverslips for further observations and photography.
In some cases movie stills were later captured using purchased DVD software, or stacks of photographs were processed using Helicon Focus software. Measurements of length and width of the tests of the specimens were made from the photographs or from drawings made with a camera lucida attached to the Nikon Optiphot microscope. The length measurement of a test was taken to be the distance between the aperture and the opposite end of the cell, whereas the width measurement was that of the widest part of the test perpendicular to the length measurement. For agglutinated domes, length and width were taken to be the widest and narrowest parts of the test, respectively. Measurements of tests are given as mean and standard deviation (SD) and t-tests were computed using Excel.
Regional overview
The area under study on the north-west coast of Scotland, bordering the east Atlantic Ocean (Figure 1), is a relatively unpolluted environment and has a mostly rocky intertidal coast with occasional small stretches of sandy beach or mudflats in enclosed bays. The underlying geology is largely that of the Dalradian (late Precambrian) Supergroup of metamorphic rocks. The examined areas were within or next to the Lynn of Lorn which extends along the east side of a large fjordic sea loch called Loch Linnhe and which includes some of the environs of two of the inner sea lochs that feed into the Lynn of Lorn, namely Loch Etive and Loch Creran. Net movement of seawater along this tract is northerly, and tidal flows with a high turbulence come from the Sound of Luing, the Gulf of Corryvreckan and the Great Race (Dale et al., Reference Dale, Boulcott and Sherwin2011).
The individual sample sites are listed in Table 1, in a north-to-south order. Loch Laich (Site 1) is a large muddy estuary (1 × 1.25 km) of a river that runs through Glen Stockdale; the estuary lies on the eastern side of Loch Linnhe and sits on a substrate of Dalradian metamorphic limestone. The most productive spots for foraminifera in Loch Laich were the mud flats that border either side of the river as it runs into the sea at low tide. Further down the coast, and next to the Lynn of Lorn, was the next sampling area, Airds Bay (Site 2), a small sandy bay covered with some sediment and with shingle on its upper reaches and bordered on one side by a metamorphic limestone outcrop. Proceeding southwards were what proved to be some of the most productive areas for foraminifera encountered in the Lorn area. The first was An Doirlinn (Site 3), an extensive muddy tidal flat about 2 km wide which faces the mouth of Loch Creran on its eastern flank and the Lynn of Lorn to the west, and which is bordered on its north side by the Isle of Eriska. This sheltered mudflat has a very gentle incline and is surfaced with sediment over a layer of fine grey clay rich with worm casts and mounds. There are only a few residential areas nearby, although Loch Creran itself possesses a number of fish farms, oyster farms, as well as a fish farm hatchery and marina. The other good sample sites associated with Loch Creran were rockpools on the promontory Sgeir Cailich (Site 4), which juts into the loch just eastwards of An Doirlinn, and a muddy bay further eastwards on the north bank of Loch Creran (Site 5), fed by a small river.
Going further southwards, two reasonably good sample areas were two remote muddy bays, Sàilean Sligeanach Bay (Site 6) and Sàilean Ruadh Bay (Site 7), fed by small streams and facing the Lynn of Lorn. Around a promontory and situated at the north side of the mouth of Loch Etive is the 3 km wide Ardmucknish Bay, bordered by a long stretch of sandy beach called Tralee and popular with holidaymakers. At the north-west end of the beach is a barrier of woods and rocks, followed by a short stretch of sandy beach, next to what proved to be a richly endowed sampling area, the mouth of a muddy estuary bordered by salt marshes and a few residences and fed by a small river (Site 8).
On the southern side of the mouth of Loch Etive was the second highly productive area, the 1 km wide Dunstaffnage Bay (Site 9), fed by a small river. At low tide the beach is muddy with plentiful worm casts and on its west side the mud gives way to gravel and pebbles. The area around this bay is more heavily populated with human activity than the other sites: going from west to east around the bay is an intact medieval castle, the buildings of the Scottish Association of Marine Science, a substantial number of residential houses and a large marina with associated moored boats. Although there is greater potential for the water here to be polluted, Dunstaffnage Bay was nevertheless one of the prime areas for finding a good selection of species of monothalamids.
It was evident that the sample areas with the highest diversity of monothalamid species were those associated with the mouths of Lochs Creran and Etive. Although I did not perform a systematic study of this phenomenon, there may be several reasons for this bounty. The two sea lochs are relatively long and thin as they were originally glaciated river valleys that have now become submerged, and each is divided into basins by shallow sills (Gage, Reference Gage1972; Lewis, Reference Lewis1988; Loh et al., Reference Loh, Reeves, Harvey, Overnell and Miller2008). A large amount of rich organic matter and other nutrients pours into the lochs via the River Creran at the head of Loch Creran, the River Etive at the head of Loch Etive and from the River Awe which flows into the middle part of Loch Etive, next to the town of Taynuilt; the River Awe is in turn supplied with large volumes of water from the freshwater Lochs Awe and Avich. The productive areas themselves are fed by small rivers, but as well as benefitting the riverine riches, they are regularly bathed by tidal flows directly from the open sea so presumably their environs do not become too brackish at any stage. The large mudflats along Loch Etive itself, and another extensive mudflat at the head of Loch Creran when sampled proved to be quite disappointing in their yield of monothalamid foraminifera, possibly because of a more brackish nature of the water due to their greater distance from the open ocean.
Terminology and systematics
The sampled species were classified as foraminifera and distinguished from Cercozoa and Gromiids by examination of live specimens for the main common specialization of foraminifera, their granulo-reticulopodia. These are characterized by a rapid bidirectional movement of intracellular organelles within a rich set of anastomosing pseudopod processes (Hedley, Reference Hedley1958; Bowser & Travis, Reference Bowser and Travis2002).
Molecular characterization has indicated that the single-chambered, soft-walled foraminifera as a whole constitute a highly diverse paraphyletic assemblage of basal lineages (Pawlowski et al., Reference Pawlowski, Holzmann and Tyszka2013). Twenty-two monothalamid marine clades, designated with capital letters, have so far been delineated by molecular phylogeny methods but these groups have not yet been classified on the basis of family and order levels (Pawlowski & Holzmann, Reference Pawlowski and Holzmann2002, Reference Pawlowski and Holzmann2008; Pawlowski et al., Reference Pawlowski, Fahrni, Brykczynska, Habura and Bowser2002a, Reference Pawlowski, Holzmann, Berney, Fahrni, Cedhagen and Bowser2002b, Reference Pawlowski, Holzmann, Berney, Fahrni, Gooday, Cedhagen, Habura and Bowser2003, Reference Pawlowski, Majewski, Longet, Guiard, Cedhagen, Gooday, Korsun, Habura and Bowser2008, Reference Pawlowski, Holzmann and Tyszka2013; Habura et al., Reference Habura, Goldstein, Broderick and Bowser2008; Pawlowski & Majewski, Reference Pawlowski and Majewski2011).
The former orders ‘Allogromiida’ and ‘Astrorhizida’ of the class Monothalamea (Haeckel, 1862, as emended by Pawlowski et al., Reference Pawlowski, Holzmann and Tyszka2013), had originally encompassed two families of monothalamids. The family ‘Allogromiidae Rhumbler, 1904’ includes monothalamids with a flexible organic wall which could serve as a cement for particles absorbed from the sediment and with one, or rarely two, terminal apertures at either end of the test, while the family ‘Astrorhizidae Brady, 1881’ embraces monothalamids with more heavily agglutinated tests. The two orders ‘Allogromiida’ and ‘Astrorhizida’, however, have not been supported by molecular phylogeny (Pawlowski et al., Reference Pawlowski, Holzmann and Tyszka2013), and so formal names for the various higher taxa of foraminifera are being avoided for the time being due to ongoing re-organization of the nomenclature for the higher levels (Anikeeva et al., Reference Anikeeva, Sergeeva and Gooday2013). All that can be said at the moment is that the specimens described in this paper belong to the following lineage on the eukaryotic Tree of Life.
Systematics
Eukaryota, Whittaker & Margulis, 1978
TSAR, Burki et al., 2020
Rhizaria, Cavalier-Smith, 2002
Retaria, Cavalier-Smith 1999
Foraminifera, d'Orbigny, 1826
Monothalamids sensu Pawlowski et al., Reference Pawlowski, Holzmann and Tyszka2013
Since no molecular characterization or study of reproduction was carried out in this investigation, and in order to prevent premature introduction of new Linnaean names into the literature, the choice was taken to refer to the specimens described here by informal names based on their gross morphology. Soft-walled, single-chambered foraminifera are divided into several morphological groupings, although when examined under a binocular microscope it is not always easy to tell these groups apart as they appear to be confounded by intermediate versions (Gooday, Reference Gooday1986, Reference Gooday2002; Voltski & Pawlowski, Reference Voltski and Pawlowski2015). The relevant morphological terminology used here is defined as follows:
1 ‘Allogromiids’ possess a transparent test wall composed of organic material mostly free of adherent particles, one or two terminal apertures and sometimes a visible invagination of the cytoplasm immediately behind the aperture referred to as a "peduncular sheath" (Siemensma et al., Reference Siemensma, Holzmann, Apothéloz-Perret-Gentil, Clauß, Voelcker, Bettighofer, Roshan, Walden, Dumack and Pawlowski2021).
2 ‘Saccamminids’ have an agglutinated test wall, with mineral particles adherent to the outside of the relatively flexible organic wall, and possess one terminal aperture or two apertures, one at each end.
3 ‘Psammophaga’ (meaning sand-eaters) are pear-shaped saccamminids that ingest mineral grains and have a single terminal aperture.
4 ‘Agglutinated spheres’ are spherical saccamminids that ingest mineral grains and have one or more indistinct apertures.
5 ‘Agglutinated domes’ rarely have visible apertures, and when removed from a substrate of stone, seaweed or shell, the thin, attached side of the test wall (if present) is left behind exposing an interior filled with cytoplasm.
The term ‘aperture structure’ is used here to refer to the differentiated part of the test that holds the aperture, and for tests with a single aperture and an obvious polarity, the part of the test that contains the aperture is the ‘apertural’ end, and the opposite side is the ‘abapertural’ end.
Results
At least 13 morphotypes of monothalamid are described below and compared with similar looking species mentioned in the published literature. A summary of where these morphotypes were found on the shorelines of the Lorn area of Western Scotland is given in Table 2. From Tables 1 and 2 it is evident that the sites numbered 1, 3, 8 and 9 had the greatest diversity of monothalamid species, corresponding to the large estuaries of Loch Laich, An Doirlinn (Loch Creran), An Sàilean (Ardmucknish Bay) and Dunstaffnage Bay (Figure 1).
Table 2. Distribution of monothalamid morphotypes at the sites numbered in Table 1

Vellaria sp.
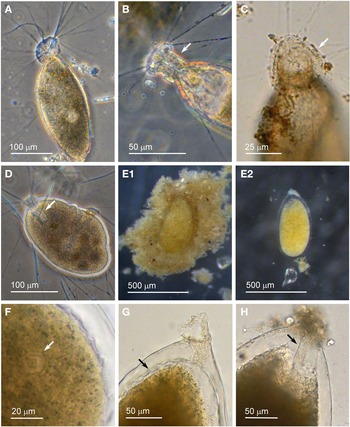
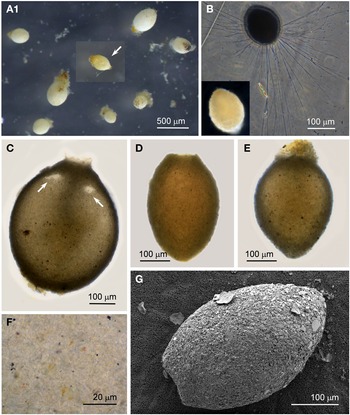
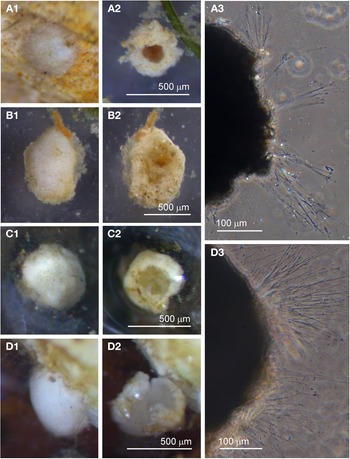
Individuals from four sites were collected with coverslip-capture and all exhibited pseudopodia characteristic of foraminifera. Dimensions for the collected specimens (N = 18) are length: 148 ± 74 μm, width: 51 ± 23 μm and length/width: 2.9 ± 0.4, indicating a slim oval shape. The test wall is largely transparent and 2–4 μm (N = 18) thick. The cytoplasm, visible through the test wall, is pale brown to white and a single nucleus 16–33 μm wide (N = 7) is visible and lies in no specific part of the cell (Figure 2A). There is a single terminal aperture structure that is a tube with a flared neck; there is no visible peduncular sheath, and occasionally a vesicle or two are visible at the base of the aperture structure (Figure 2B). The flared structure at the aperture end is very thin: it appears dark in phase contrast (Figure 2A) and possesses some granular detail (Figure 2C). There was some variation in test shape and appearance of the aperture structure among the collected specimens, suggesting there may have been more than one species present in the group. The test was symmetrically oval in the majority, wider at the apertural end in two of the specimens and wider at the abapertural end in the remaining three. There was not enough morphological demarcation between the cells, however, for an obvious division into subtypes.

Fig. 2. Vellaria sp.: (A) pseudopodia; (B) aperture structure (arrowed); (C) granular appearance (arrowed) of aperture structure. Undetermined monothalamid 1: (D) pseudopodia, wrinkled test wall and peduncular sheath (arrowed). Allogromiid sp. 1: (E1) partially encased within a feeding cyst; (E2) after removal from feeding cyst. Allogromiid sp. 1 (glycerol-embedded): (F) granules and an unknown structure (arrowed) within the cytoplasm, possibly a nucleus; (G) corrugated appearance of the surface of the cytoplasm (arrowed), and aperture structure; (H) peduncular sheath (arrowed).
The Vellaria sp. described here was only able to be recovered by coverslip-capture, a method which works well with small, transparent monothalamids that are difficult to spot by direct observance of the sediment. It could be argued that the small Vellaria sp. specimens described here were all juveniles, except that during the time period of this study no larger counterparts were seen by direct examination of sediments from various sites and at different times of the year.
Comments
The type species name for the genus Vellaria appears as either Vellaria pellucidus or V. pellucida in the literature (Gooday & Fernando, Reference Gooday and Fernando1992; Gooday et al., Reference Gooday, Anikeeva and Pawlowski2010) and as V. pellucida in the ‘World Register of Marine Species’, so it is referred to here by the latter name. Vellaria pellucida Gooday & Fernando, Reference Gooday and Fernando1992 was first observed in the Vellar estuary in the Bay of Bengal, India (Gooday & Fernando, Reference Gooday and Fernando1992; Gooday, Reference Gooday2002), and a Vellaria pellucida was reported to be at depths of 5–10 m in the Black Sea (Gooday et al., Reference Gooday, Anikeeva and Pawlowski2010, figure 7). As with the Vellaria sp. of the current study, the published Vellaria species have a transparent test wall with no agglutinated particles attached, no peduncular sheath, and an aperture structure that is a tube with a flared ending with mineral grains attached to its outer margin. The dimensions are different: Vellaria pellucida from the Vellar estuary is considerably more bulky and is stouter, with length: 295 ± 63 μm, width: 138 ± 32 μm, length/width: 2.1 ± 0.3 and test wall 7–10 μm thick. The Black Sea version is also stouter, with the same length/width ratio of 2.1 ± 0.4 as the Indian variety, but it is closer in size to the current Vellaria sp. with length: 220 ± 48 μm, width: 108 ± 23 μm and test wall 2–3 μm thick. No molecular data have been obtained for V. pellucida from the Indian locality, but molecular characterization of the V. pellucida from the Black Sea indicates that is related to those in the genus Psammophaga Arnold, Reference Arnold1982. A morphologically uncharacterized Vellaria sp. (HE998684) was placed in Clade E of monothalamids, sister to the ‘sand-eater’ Psammophaga sp. HE998686 (Pawlowski et al., Reference Pawlowski, Holzmann and Tyszka2013).
Undetermined monothalamid 1
Individuals from five sites were observed with coverslip-capture and all had pseudopodia characteristic of foraminifera. Dimensions for the collected specimens (N = 6) are length: 239 ± 76 μm, width: 156 ± 45 μm and length/width: 1.5 ± 0.2, indicating a plump oval shape. The test wall has folds giving it a wrinkled appearance and is wholly or partially transparent and 5–8 μm (N = 6) thick (Figure 2D). The distorted outline of the test wall did not seem to be due to damage, because all six specimens looked healthy and were active. The cytoplasm, visible through the test wall, is pale brown, occasionally with remains of prey visible inside; under higher power the cytoplasm can be seen to be churning about. A single nucleus 25–45 μm (N = 5) is sometimes visible in the central or abapertural end of the cell. There is a single terminal aperture structure shaped like a tube and it is continuous with a peduncular sheath 16–28 μm (N = 4) wide (Figure 2D).
Comments
The only counterpart to the Undetermined monothalamid 1 described above is the single Undetermined allogromiid ICEMON7 specimen found in shallow waters around Iceland (Voltski & Pawlowski, Reference Voltski and Pawlowski2015, plate 2/C). This monothalamid had some of the same morphological features as the ones described here: a flexible, folded transparent test wall of ~6 μm and a single aperture continuous with a peduncular sheath of ~20 μm diameter, but it was smaller, with a test of length 175 μm and width 127 μm. The folded test wall of this individual was regarded by the authors as a deformity due to handling, but the six specimens in my own study had all been isolated by coverslip-capture and not picked up with a brush, so the folded test wall is likely to be a natural feature of this type of species. Molecular characterization of Undetermined allogromiid ICEMON7 placed it at the base of Monothalamid Clade Y (Voltski & Pawlowski, Reference Voltski and Pawlowski2015).
Allogromiid sp. 1
Specimens, all initially enclosed in muddy cyst-like structures (Figure 2E), were found at three sites and the majority were from Dunstaffnage Bay. One specimen was attached to a piece of seaweed but the remainder were living in the sediment. The muddy-looking cyst wall has two layers, a loose outer later that is easily removed and a slightly tougher inner layer. The dissection process required to free the specimens from the cysts may have damaged some of them, because only 14 out of the 22 specimens displayed pseudopodia, all of the type typical of foraminifera. Dimensions for the tests (N = 22) are length: 421 ± 80 μm, width: 269 ± 47 μm and length/width: 1.6 ± 0.3, indicating a plump oval shape. The test wall is highly reflective, wholly transparent and rather thick: 12–24 μm (N = 19) wide. In some of the larger specimens, particles of mud from the enveloping cyst remained attached in clumps to the outer surface of the test which seems to be rather sticky. The cytoplasm is a uniform pale yellowish-green (Figure 2E2) and a single nucleus 41–57 μm (N = 7) wide is visible in no particular part of the cell. In glycerol-embedded specimens observed under high power, numerous tiny granules of uniform size and shape are seen in the cytoplasm (Figure 2F). The embedding in glycerol causes the cytoplasm to pull away from the test wall so that the outer surface of the cytoplasm, visible through the transparent test wall acquires a scalloped or corrugated surface (Figure 2G). The terminal aperture structure is a thin-walled tube that is continuous with a peduncular sheath 25–39 μm (N = 11) wide (Figure 2G, H). An unusual feature of the pseudopodia is that as well as probing the medium in the usual way, they also wrap themselves around the test, and this may be the mechanism used for building its protective layer of muddy sediment.
Comments
Allogromiid sp. 1 bears a certain resemblance to members of the genus Allogromia Rhumbler, Reference Rhumbler1904. A species with a peduncular sheath termed Gromia lagenoides (Gruber, Reference Gruber1884), was first described and illustrated by Gruber (Reference Gruber1884, figure 17). It has since been renamed Allogromia lagenoides (Gruber, Reference Gruber1884) because members of the genus Gromia are not foraminifera. Various species of Allogromia were illustrated by Rhumbler (Reference Rhumbler1904) including A. ovoidea Rhumbler, Reference Rhumbler1904 (figure 18), type species A. mollis (Gruber, Reference Gruber1884, figure 21) and A. lagenoides (Gruber, Reference Gruber1884, figure 20, a copy of figure 17 of Gruber, Reference Gruber1884). A key for identification of the species provided by Rhumbler (Reference Rhumbler1904) suggests that Allogromiid sp. 1 keys out as being most closely related to A. lagenoides, with an egg-shaped test, a test wall ‘as clear as glass’ that is very thick but quite soft and pliable and sometimes sprinkled with grains of sand, a peduncular sheath but no other internal structure visible inside the cell, and strong pseudopodia that radiate out in a rich tuft, usually directed forward from the mouth but with a few that rest against the test wall. Allogromia lagenoides, however, is much smaller than Allogromiid sp. 1, 80 μm long and 60 μm wide and there is no mention of the existence of an enveloping feeding cyst.
Allogromiid sp. 1 also has certain morphological similarities to Allogromia laticollaris Arnold, Reference Arnold1948, first isolated from the littoral zone of the Bay of St. Andrews at Panama City, Florida (in Arnold, Reference Arnold1948, plate I/1). The most distinctive features that Allogromiid sp. 1 has in common with A. laticollaris are a transparent test wall, peduncular sheath and granulo-reticulopodia that attempt to envelope the test, but A. laticollaris is slightly smaller, 100–450 μm long and with a much thinner wall that is 4–6 μm thick. The cytoplasm of A. laticollaris described by Arnold (Reference Arnold1948) was of a deep orange colour but this may reflect the nature of the prey as A. laticollaris was full of the shells of diatoms and other unicellular algae and there was no mention of a muddy feeding cyst. The fact that Allogromiid sp. 1 has no visible prey inclusions may be because it feeds on bacteria instead, and the role of the feeding cyst may be to harvest the bacteria from the external medium. A molecular determination of a specimen A. laticollaris has indicated that it branches within Monothalamid Clade M (Pawlowski et al., Reference Pawlowski, Holzmann, Berney, Fahrni, Cedhagen and Bowser2002b).
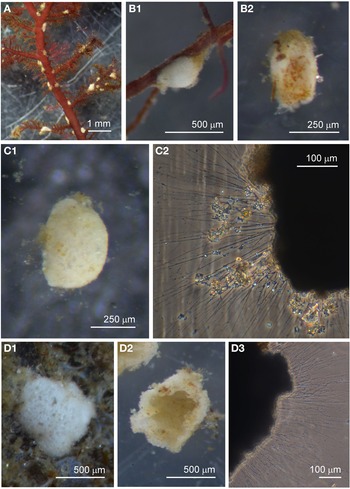
Allogromiid sp. 2
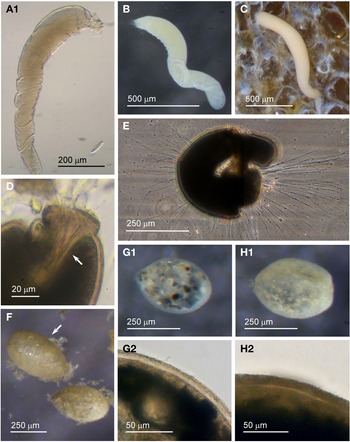
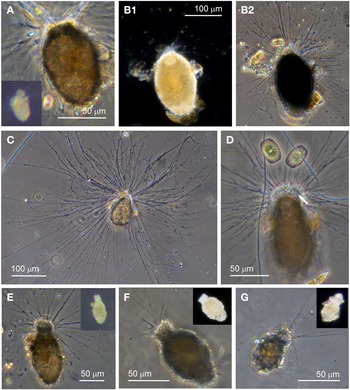
Examples of this allogromiid were collected from three sites where they were associated with seaweed, and all collected were active (N = 5). The dimensions for the collected specimens are length: 981 ± 368 μm, width: 154 ± 29 μm and length/width: 6.3 ± 1.8, indicating a highly elongated shape. The test wall is highly flexible and is 3–5 μm (N = 3) thick. In glycerol-embedded specimens the test wall appears transparent (Figure 3A) but in live specimens the test wall has a matt surface (Figure 3B, C) due to a finely agglutinated outer layer of the test wall (Figure 3D). The cytoplasm is creamy white with no visible food vacuoles (Figure 3A–C), and in their natural state the cells lie curled around the blades of red seaweed such as Ceramium virgatum or lie flat on blades of kelp (Figure 3C). In glycerol-embedded specimens numerous doughnut-shaped inclusions 21–23 μm wide (N = 3), which may be nuclei, are visible within the cytoplasm. Vast numbers of minute, cylindrical-shaped granules of uniform size are also scattered in the cytoplasm. The terminal aperture structure was usually obscured by sediment but was exposed in one specimen, and is in the form of a collar attached to a 19 μm wide peduncular sheath (Figure 3D). Although some of the specimens looked as if they had been damaged by handling, they all emitted a rich array of pseudopodia (Figure 3E).

Fig. 3. Allogromiid sp. 2 (glycerol-embedded): (A) with light brown cytoplasm. Allogromiid sp. 2: (B) detached from red seaweed; (C) another specimen on a blade of kelp; (D) aperture structure with peduncular sheath (arrowed); (E) pseudopodia. Psammophaga sp.: (F) two specimens, one with a transversely ridged test wall (arrowed). Psammophaga sp. (glycerol-embedded): (G1–G2) specimen with mineral particles clearly visible within and a double-layered, lightly agglutinated test wall; (H1–H2) specimen with mineral particles partially obscured by a heavily agglutinated test wall.
Comments
Allogromiid sp. 2 has many of the same features as type species Dactylosaccus vermiformis Rhumbler, 1894, especially the way it lies naturally curled up (Rhumbler, Reference Rhumbler1904, Figure 17), but D. vermiformis is much larger, 4 mm long and 0.34 mm wide. A closer morphotype to Allogromiid sp. 2 is the more recently discovered type taxon Bowseria arctowskii Sinniger et al., Reference Sinniger, Lecroq, Majewski and Pawlowski2008 from Admiralty Bay, King George Island, West Antarctica (Sinniger et al., Reference Sinniger, Lecroq, Majewski and Pawlowski2008, Figure 2). The details of B. arctowskii that are similar to those of Allogromiid sp. 2 are an elongate, tubular, often slightly curved and irregularly shaped test with an apertural end sometimes wider and more broadly rounded compared with the narrower distal end, a single terminal aperture continuous with an internal structure and a white to yellowish cytoplasm full of fine granules. Bowseria arctowskii is slightly larger, however, ranging from 1–2 mm long and between 0.125–0.350 mm wide and the test wall is much thinner at about 1 μm, wholly transparent and with a distinctively shiny, reflective surface. These differences between B. arctowskii and Allogromiid sp. 2 may reflect to the different habitats they occupy as B. arctowskii lives in much deeper colder waters. Allogromiid sp. 2 was only occasionally encountered and only found on seaweed, so it may actually be more abundant in a deeper photic zone. The molecular characterization of Bowseria arctowskii by Sinniger et al. (Reference Sinniger, Lecroq, Majewski and Pawlowski2008) indicated that it is closely related to a species of the unlikely genus Psammosphaera Schulze, 1875. Both a Psammosphaera sp. and a Bowseria sp. were subsequently allocated to Monothalamid Clade B (Voltski & Pawlowski, Reference Voltski and Pawlowski2015).
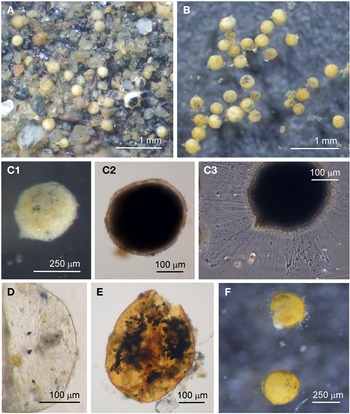
Psammophaga sp.
Specimens were collected from three sites, the majority by magnet capture from sediment collected from Dunstaffnage Bay. The species was particularly abundant during the autumn months. Dimensions for the collected specimens (N = 125) are length: 423 ± 66 μm, width: 242 ± 35 μm and length/width: 1.8 ± 0.2, indicating an oval or pyriform (pear-shaped) outline. Under incident light, the test wall of live specimens looks grey or light brown and exhibits various degrees of agglutination, and transverse ridging is sometimes visible on the surface of the test wall (Figure 3F). In specimens embedded in glycerol there appears to be a differentiation into a ‘lightly agglutinated’ form (Figure 3G1) and a ‘heavily agglutinated’ form (Figure 3H1). In each type, the test wall is made up of two clearly demarcated layers, an outer agglutinated layer and a translucent inner layer lacking in granules. The outer layer in the ‘lightly agglutinated’ test is 2–4 μm thick (N = 13) and the inner layer is 5–7 μm thick (Figure 3G2), and for the ‘heavily agglutinated’ test the values are 5–9 and 3–9 μm (N = 9), respectively (Figure 3H2). Other than this, there are no obvious morphological distinctions between the two types of specimen.
The cytoplasm visible through the test wall is creamy white, and embedded within are numerous mineral granules, some of them very dark and presumed to be magnetite. The specific gravity of magnetite is twice that of the common mineral quartz, and the darker granules tended to rest on the lower side of the cell as it lies in the sediment (Figure 4A). The dark granules, more clearly visible in glycerol-embedded specimens, are also not always evenly distributed along the soma. They may congregate either at the apertural end of the cell (Figure 4B), at the abapertural end (Figure 4C) or they are distributed along the length of the cell (Figure 4D).

Fig. 4. Psammophaga sp.: (A1) specimen with mineral particles obscured by a heavily agglutinated test; (A2) same specimen turned over to show dark mineral particles concentrated on the reverse side of the cell body. Psammophaga sp. (glycerol-embedded): specimen with dark mineral particles congregated at the (B) abapertural end, (C) apertural end, (D) or evenly distributed throughout; (E) simple aperture structure with dark mineral particles within; (F) aperture structure with a large mineral particle within. Psammophaga sp.: (G) relatively differentiated aperture structure; (H) pseudopodia.
All cells have a single terminal aperture structure mostly of a relatively simple construction (Figure 4E, F) but sometimes the aperture structure has a more differentiated appearance (Figure 4G). In some specimens the dark mineral particles appear to be making their way through the aperture into the interior of the cell (Figure 4E, F). Specimens that are active possess pseudopodia characteristic of foraminifera (Figure 4H), although the processes are relatively sparse compared with those of some of the other species of monothalamid encountered.
Comments
The specimens described here have all the features of the genus Psammophaga Arnold, Reference Arnold1982. The type species for the genus is Psammophaga simplora Arnold, Reference Arnold1982, from shallow waters of Monterey Bay, California. It is described as having a pyriform to ovoid test of 0.25 mm in length, a flexible wall of a thick transparent matrix with a thinner outer agglutinated covering; the aperture is terminal, simple and at the pointed end of the test or may be slightly produced on a neck and there is no peduncular sheath. Its most distinctive feature is the presence of numerous mineral particles inside the test (Arnold, Reference Arnold1982), hence the name Psammophaga, or ‘sand-eater’. Other members of the Psammophaga genus have been found all over the world, mainly in shallow water. All those that have been described are in the size range of 200–600 μm and lack enough characteristic features for them to be distinguished into separate species on the basis of morphology alone. Examples are (1) Psammophaga crystallifera (Dahlgren, Reference Dahlgren1962a) from the Gullmar Fjord on the west coast of Sweden (Dahlgren, Reference Dahlgren1962a) and an intertidal mudflat near Southampton (Larkin & Gooday, Reference Larkin and Gooday2004, plate 1/1, 2), Psammophaga sp. from Explorer's Cove Antarctica (Gooday et al., Reference Gooday, Bowser and Bernhard1996, plate 4/4) and (3) Psammophaga cf. P. simplora in mudflats and tidal creeks from Sapelo Island area, Georgia (Habura et al., Reference Habura, Goldstein, Broderick and Bowser2008, figure 2F). Two species with magnetic particles within are (1) Psammophaga magnetica Pawlowski & Majewski, Reference Pawlowski and Majewski2011 from Admiralty Bay, West Antarctica (Pawlowski & Majewski, Reference Pawlowski and Majewski2011, figure 2) and (2) Psammophaga zirconia Sabbatini et al., Reference Sabbatini, Negri, Bartolini, Morigi, Boudouma, Dinelli, Florindo, Galeazzi, Holzmann, Lurcock, Massaccesi, Pawlowski and Rocchi2016 from the Adriatic Sea (Sabbatini et al., Reference Sabbatini, Negri, Bartolini, Morigi, Boudouma, Dinelli, Florindo, Galeazzi, Holzmann, Lurcock, Massaccesi, Pawlowski and Rocchi2016, figures 2, 3). It is not known whether the presence of magnetic particles is a feature of the geology of the environment or a species-specific quality.
The presence of magnetic particles in the Psammophaga sp. described here made it possible to use a small magnet to isolate large numbers of individuals for study. As a result it was noted that there were cells with tests of various levels of thickness and agglutination, suggesting more than one species was represented in the collection. Psammophaga species with different levels of agglutination are represented by the following: (1) Psammophaga fuegia Gschwend et al., Reference Gschwend, Majda, Majewski and Pawlowski2016 from the Beale Channel, South America (Gschwend et al., Reference Gschwend, Majda, Majewski and Pawlowski2016, figures 2, 3), (2) Psammophaga sp. 1–4 from the Western Svalbard fiords (Majewski et al., Reference Majewski, Pawłowski and Zajaczkowski2005, figure 2/11–15), (3) Psammophaga sp. from the intertidal mudflat near Southampton (Larkin & Gooday, Reference Larkin and Gooday2004, plate 1/3 and 4), (4) Psammophaga sp. #209–212 with magnetic properties from Admiralty Bay, King George Island, West Antarctica (Majewski et al., Reference Majewski, Lecroq, Sinniger and Pawlowski2007, figure 2/3), (5) Psammophaga sp. from the Crimean Peninsula, Black Sea (Gooday et al., Reference Gooday, Anikeeva and Pawlowski2010, figure 8) and (6) Psammophaga sapela Altin-Ballero et al., Reference Altin-Ballero, Habura and Goldstein2013, also with magnetic properties, from coastal Georgia (Altin-Ballero et al., Reference Altin-Ballero, Habura and Goldstein2013, figure 2).
In molecular phylogenetic studies many Psammophaga sequences have been shown to cluster together in Clade E and to form a sister group to species of Vellaria (Pawlowski et al., Reference Pawlowski, Holzmann, Berney, Fahrni, Cedhagen and Bowser2002b, Reference Pawlowski, Holzmann and Tyszka2013). Psammophaga species are not all closely related, and a molecular study on Psammophaga species collected from a depth of 30–60 m near Dunstaffnage Bay, not characterized morphologically, were found to be related closely to undetermined Psammophaga species from Svalbard and to P. magnetica from Antarctica but more distantly related to P. fuegia, P. sapela and P. crystallifera (Pawlowski & Holzmann, Reference Pawlowski and Holzmann2008; Pawlowski et al., Reference Pawlowski, Majewski, Longet, Guiard, Cedhagen, Gooday, Korsun, Habura and Bowser2008; Pawlowski & Majewski, Reference Pawlowski and Majewski2011; Gschwend et al., Reference Gschwend, Majda, Majewski and Pawlowski2016). The uncharacterized species from Dunstaffnage Bay may be the same or close relatives of one or two of the Psammophaga variants described here that were abundant on the intertidal zone of Dunstaffnage Bay.
Saccamminid sp. 1
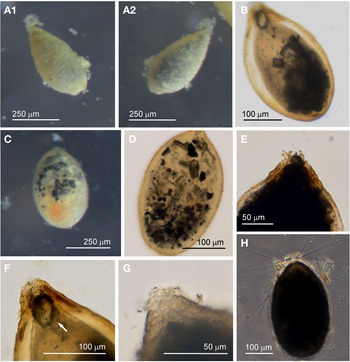
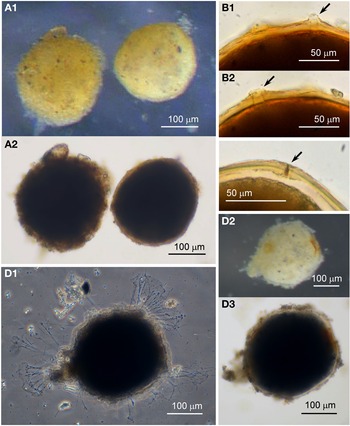
Specimens (N = 53) were collected from the sediment from five sites and they were most prolific in the spring months. The test of this morphotype has a broad oval shape, oval, widest near the midpoint and often has a somewhat pointed abapertural end (Figure 5A–E). In live individuals the pseudopodia are relatively profuse (Figure 5B). The tests of specimens collected during the spring months have a matt white appearance resembling pure alabaster, but they are not soluble in HCl. In the autumn months, when the numbers of specimens have declined, the abapertural ends of the tests acquire an orange colour (Figure 5A, arrowed). The test wall of all specimens is relatively opaque, and even when embedded in glycerol, no internal structures are visible and there is no sign of a peduncular sheath (Figure 5C–E). None of the examined specimens were magnetic indicating that Saccamminid sp. 1 is not in the habit of ingesting mineral particles. The test wall is covered with small flaky mineral granules (Figure 5F, G); most are colourless flakes, but there is a sprinkling of smaller orange- and black-coloured granules among them (Figure 5F). Although the test wall appears to be rigid under reflected light, it is nevertheless flexible in that it may buckle in some specimens when preserved in alcohol and embedded in glycerol. There is a single terminal aperture structure that is a simple opening (Figure 5D, E) but sometimes in addition there is flared extension made of organic material (Figure 5C).

Fig. 5. Saccamminid sp. 1: (A) specimens of various shapes and sizes, with sediment concentrated around the apertures; the test of the specimen in the inset (arrowed) has orange pigmentation at the abapertural end; (B) pseudopodia. Saccamminid sp. 1 (glycerine-embedded): (C) holes in the agglutination of the wall (arrowed) in a Type 2 specimen; (D & E): examples of aperture structure and test shape; (F) mineral particles in the agglutinated test wall; (G) scanning electron micrograph of the test wall (courtesy of J. Poole).
There are two subtypes of test for Saccamminid sp. 1, Type 1 (N = 43) and Type 2 (N = 10). They are identical in all respects except that the second type possesses what appear to be holes in the test wall distributed in a ring around the aperture structure. When viewed in glycerol under high magnification, it is evident that the ‘holes’ in the test wall are simply where agglutination particles have eroded away or are absent (Figure 5C). Whereas 40/43 of the Type 1 cells exhibited granulo-reticulopodia characteristic of the foraminifera, only one specimen from Type 2 was active, suggesting that most of the Type 2 tests may have developed the holes post mortem or belonged to aged specimens. There was also a difference in size between the two types: the dimensions for the Type 1 tests are length: 335 ± 59 μm, width: 235 ± 48 μm and length/width: 1.4 ± 0.1 and for the Type 2 tests are length: 464 ± 45 μm, width: 342 ± 60 μm and length/width: 1.4 ± 0.2. The Type 2 tests are significantly longer and broader than Type 1 (two-tailed t-test P < 0.01).
Comments
The features of Saccamminid sp. 1 fit quite closely to those of four specimens found in shallow water near Plymouth by Heron-Allen & Earland (Reference Heron-Allen and Earland1930, plate III/figures 34–35) and given the name Hippocrepina pusilla Heron-Allen & Earland, Reference Heron-Allen and Earland1930, type species: Hippocrepina indivisa Parker, 1870. The test of H. pusilla is described as being rounded at the oral extremity and tapering to an acute point at the other end, with a circular aperture variable in size and a wall that is covered with minute scales of mineral material, and of length 550 μm and width 370–400 μm. The colour of the test is described as being of ‘a lustrous grey at the oral end, gradually deepening to rusty brown at the aboral extremity’.
Saccamminid sp. 1 also has a close likeness to type species Cedhagenia saltatus Gooday et al., Reference Gooday, Anikeeva and Pawlowski2010 from the Crimean Peninsula, Black Sea (Gooday et al., Reference Gooday, Anikeeva and Pawlowski2010, figures 4, 5, 6). The test of C. saltatus is more translucent when embedded in glycerol and is smaller, 218 ± 30.4 μm long, 104 ± 18.7 μm wide with a length/width ratio of 2.12 ± 0.25. Under scanning electron microscopy the surface features of the test wall are very similar in both species, although the test wall of Saccamminid sp. 1 does not become flattened under these conditions but either remains rounded or it breaks up; this could be due to differences in the flexibility of the test walls or due to the way the specimens were prepared in each laboratory. Another species of similar appearance and size to Saccamminid sp. 1 is type taxon Ovammina opaca Dahlgren, Reference Dahlgren1962b from the Gullmar Fiord (Dahlgren, Reference Dahlgren1962b), see plate 22/figures 7–11 of Loeblich & Tappan (Reference Loeblich and Tappan1988). Saccamminid sp. 1 is nevertheless much larger than O. opaca and lacks a peduncular sheath. Interestingly, molecular characterization of the similar looking species Cedhagenia saltatus and Ovammina opaca (Gooday et al., Reference Gooday, Anikeeva and Pawlowski2010) has indicated that they are closely related and that they are both situated in Monothalamid Clade O (ilustrated in figure 1 of Voltski & Pawlowski, Reference Voltski and Pawlowski2015).
Saccamminid sp. 2
Specimens (N = 54) were collected from six sites and the main feature they have in common is an opaque, white or yellowish-white, vase-shaped test. There was enough of a variety of test profiles and wall textures, nevertheless, to suggest that this was a heterogeneous group of species that do not have features distinct enough for them to be separated into individual subclasses (Figure 6A). Many specimens were active and those that were gave rise to a rich network of granulo-reticulopodia (Figure 6B). Dimensions for the collected specimens are length: 380 ± 74 μm, width: 184 ± 38 μm and length/width: 2.1 ± 0.3 indicating a slim oval shape, although some were sub-cylindrical rather than oval in shape. The tests are mostly white, some with yellow colouring at the abapetural end or throughout. In a proportion of the specimens the test wall is very reflective and has a silvery sheen, with the colour and appearance of raw silk, but for others the test wall is more matt in appearance. There is a single terminal aperture normally covered with sediment, but when visible, has a rim like a collar or a short tube.

Fig. 6. Saccamminid sp. 2: (A) assorted shapes and colours of tests; (B1 & B2) pseudopodia of a vase-shaped individual.
Comments
Saccamminid sp. 2 individuals bear some resemblance to the species Hippocrepinella alba Heron-Allen & Earland, Reference Heron-Allen and Earland1932b, renamed type taxon Cribrothalammina alba Heron-Allen & Earland, Reference Heron-Allen and Earland1932b. This was illustrated by Heron-Allen & Earland (Reference Heron-Allen and Earland1932b: plate I, figures 16–18) and described as having a test that is ‘uniformly white, cylindrical or fusiform in shape, furnished with a large principal aperture with or without a collar, sometimes with apertures at both ends, and with a test wall that is very smooth and agglutinated with very minute particles’. Cribrothalammina alba is much larger than Saccamminid sp. 2, 0.5–2.8 mm long and 0.09–0.3 mm broad, and it was suspected by Heron-Allen & Earland that their collection may have harboured more than one species.
Saccamminids like the Saccamminid sp. 2 with the silvery test have been described recently but were either larger, extremely elongated, or with apertures at both ends, and were encountered in the much colder environment of Explorer's Cove, Antarctica (Gooday et al., Reference Gooday, Bowser and Bernhard1996), the West Spitsbergen fjords, Svalbard (Majewski et al., Reference Majewski, Pawłowski and Zajaczkowski2005, figures 3/3, 3/6), under the Ross ice shelf in Antarctica (Pawlowski et al., Reference Pawlowski, Fahrni, Guiard, Conlan, Hardecker, Habura and Bowser2005, figure 1F) and in the South Georgia fjords (Holzmann et al., Reference Holzmann, Gooday, Majewski and Pawlowski2022). Molecular characterization of two silver saccamminids from the Antarctic has indicated that they are closely related to a species Hippocrepinella alba from the same habitat (Pawlowski et al., Reference Pawlowski, Fahrni, Guiard, Conlan, Hardecker, Habura and Bowser2005).
Saccamminid sp. 3
One single large, live specimen was found attached to a piece of fine seaweed. It has a yellowish reflective surface (Figure 7A) and profuse pseudopodia typical of the foraminifera (Figure 7B). Dimensions for the single specimen are length: 1095 μm, width: 442 μm and length/width: 2.5 indicating a broad, elongated shape. The aperture was obscured in the live specimen by sediment, but preservation in alcohol and clearance in glycerol revealed a tube-shaped terminal aperture with thick walls (Figure 7C). The specimen was damaged by the preservation and embedding procedures and some of the test wall had sloughed off, showing it has an agglutination made of very fine particles (Figure 7D). The test wall seen in profile in the main part of the specimen has an 8 μm thick organic layer overlain by a finely agglutinated 5 μm thick outer layer (Figure 7E). The colour of the organism is due to the yellow-coloured cytoplasm which was extruded when the specimen was damaged (Figure 7C).

Fig. 7. Saccamminid sp. 3: (A) live individual with sediment at the aperture end; (B) pseudopodia. Same specimen after glycerol-embedding (slightly damaged): (C) aperture and yellowish cytoplasm; (D) detail of agglutinated test wall; (E) double-layered test wall (arrowed) seen from the side.
Comments
Saccamminid sp. 3 has a close likeness to type species Phainogullmia aurata Nyholm, Reference Nyholm1955 from the Gullmar Fjord (Nyholm, Reference Nyholm1955), illustrated by Loeblich & Tappan (Reference Loeblich and Tappan1988, plates 8/5, 8/6) and described as having a cylindrical test 0.2–1.4 mm in length with a glossy yellowish-brown, opaque but flexible test wall with an aperture at each end of test from which protrude reticulate pseudopodia. Saccamminid sp. 3 is also identical in appearance to, but much smaller than Phainogullmia cf. aurata of Admiralty Bay, King George Island, West Antarctica (Majewski et al., Reference Majewski, Lecroq, Sinniger and Pawlowski2007, figure 4/3).
Undetermined monothalamid 2
Live individuals were recovered with the coverslip-capture method from six sites. Dimensions for the collected specimens (N = 33) are length: 93 ± 37 μm, width: 56 ± 18 μm and length/width: 1.6 ± 0.3, indicating an oval shape (Figure 8A–D). The test wall is white to light brown, heavily agglutinated and therefore opaque. There is a single simple terminal aperture, sometimes with a narrow collar-like extension, from which issue a set of remarkably profuse pseudopodia with type features of the granulo-reticulopodia of foraminifera. In some specimens the cytoplasm protrudes in a mass in addition to the regular pseudopodia (Figure 8D).

Fig. 8. Undetermined monothalamid 2: (A–D) appearances and pseudopodia of four specimens, sometimes including a protoplasmic mass (arrowed). Undetermined monothalamid 3: (E–G) appearances and pseudopodia of three specimens.
Comments
The specimens described here, only seen with coverslip-capture due to their small size, are similar in some respects but much smaller than the Saccamminid sp. 1 described above, so they could well be immature versions of it. Undetermined monothalamid 2 is also comparable in size and appearance to the undetermined Saccamminid ICEMON3 from Iceland waters that has a molecular affinity to Ovammina opaca and Cedhagenia saltatus and which belongs to Monothalamid Clade O (Voltski & Pawlowski, Reference Voltski and Pawlowski2015, figure 2D, E).
Undetermined monothalamid 3
Live individuals were recovered by coverslip-capture from three sites. Dimensions for the collected specimens (N = 8) are length: 72 ± 26 μm, width: 41 ± 11 μm and length/width: 1.7 ± 0.3, indicating an oval shape (Figure 8E–G). The test wall is white to light brown, heavily agglutinated and therefore opaque. The agglutinated particles are coarser that in the saccamminid 2 and so its surface is rough-looking (Figure 8E, F), especially in the smaller specimens (Figure 8G). There is a single terminal apertural structure with a flared, funnel-like shape from which issues a set of pseudopodia (Figure 8E–G) that are not as profuse as that for the Undetermined monothalamid 2.
Comments
Undetermined monothalamid 3 was seen only by coverslip-capture, and the small size of this species (or several species) merits it being placed in the ‘micro-foraminifera’ category of foraminifera which are smaller than 100 μm in length (Pawlowski et al., Reference Pawlowski, Lee and Gooday1993; Gooday et al., Reference Gooday, Carstens and Thiel1995). Undetermined monothalamid 3 is similar in appearance and size to those in the genus Lagenammina Rhumbler, Reference Rhumbler1911; type taxon Lagenammina laguncula Rhumbler, Reference Rhumbler1911, from the North-east Atlantic abyssal sediments (Gooday et al., Reference Gooday, Carstens and Thiel1995, plate I/E, F). The Lagenammina species described by Loeblich & Tappan (Reference Loeblich and Tappan1988, plate 21/8 and 9) has a flask-shaped test with a proteinaceous organic layer densely covered by agglutinated material and an elongated, pointed terminal aperture structure, illustrated also by Rhumbler (Reference Rhumbler1911, plate II). In the Undetermined monothalamid 3, however, the aperture structure is not so elongated and it has a flared ending. Because of this particular feature it has a resemblance to type taxon Ovulina urnula Gruber, Reference Gruber1884, illustrated in Gruber (Reference Gruber1884, figure 9), and described as having a white agglutinated test, 0.15 mm long and 0.1 mm wide and resembling a ‘delicate little urn or even a balloon’, the back end of the test being enlarged while at the front there is a neck-like aperture structure with a flared opening. Undetermined monothalamid 3 is also similar in size and appearance, but less translucent, than undetermined allogromiid ICEMON1 from Iceland waters (Voltski & Pawlowski, Reference Voltski and Pawlowski2015, figure 2A).
Agglutinated sphere
Specimens were collected from two sites, the majority by magnet capture from sediment in Dunstaffnage Bay. They were more commonly observed during the autumn months. Dimensions for the collected Agglutinated spheres (N = 270) are length: 313 ± 64 μm, width: 294 ± 64 μm and length/width: 1.1 ± 0.1, indicating a largely spherical shape (Figure 9A, B). Those that are active exhibit pseudopodia characteristic of foraminifera (Figure 9C). The cytoplasm is opaque and with ingested mineral within, and some of the mineral granules are black, possibly made of magnetite. As with the Psammophaga sp., the dark grains are located close to the underside of the cell side as it rests on a substrate, so that the cell has to be turned over to reveal the presence of the black granules within the cytoplasm (Figure 9B). It was confirmed that the dark particles are within the cytoplasm rather than embedded on one side of the test wall because the dark granules are visible in extruded cytoplasm and not in the walls of a test from which the cytoplasm has been removed (Figure 9D). Sometimes empty tests are found in the sediment in which the cytoplasm has degraded naturally, leaving magnetic granules inside the otherwise empty cavity of the test (Figure 9E).

Fig. 9. Agglutinated sphere: (A) after magnet capture; (B) turned over to reveal dark mineral particles on the reverse side of the body; (C1–C3) appearance and pseudopodia. Agglutinated sphere (glycerol-embedded): (D) empty test with light agglutination; (E) empty test in which the cytoplasm has decayed naturally, leaving behind the dark mineral particles within; (F) two specimens with orange-coloured tests and white cytoplasm within.
The Agglutinated sphere species display a range of colouration from colourless to orange-coloured (Figure 9). The pigment is in the test wall, probably due to incorporated iron compounds, and is not due to the colour of the cytoplasm which is seen to be of a creamy white colour when extruded from the orange-coloured tests (Figure 9F, upper cell).
Examination of a group of cells that had been preserved in alcohol and cleared in glycerol reveals further details. The test wall is a single layer of transparent organic material to which is loosely attached various quantities of fine and coarse transparent mineral granules, giving the superficial appearance of cells with a range of lightly to heavily agglutinated tests (Figure 10A). None of the granules showed any reaction in HCl. Wall thickness in the more lightly agglutinated form (N = 25) ranges from 3–11 μm and in the heavily agglutinated form (N = 6) from 6–12 μm. Some of the specimens had lost their granules in patches, exposing the transparent test wall and the milky cytoplasm within that is opaque even in glycerol.

Fig. 10. Agglutinated sphere (glycerol-embedded): (A1 & A2) two cells with different levels of agglutination of the test walls; (B) aperture structures (arrowed); (C) mineral grain (arrowed) in an aperture structure. Agglutinated sphere: (D1–3) a specimen with pseudopodia arising from different points in the test wall.
Under high power, the test walls of glycerol-embedded cells are seen to possess at least one simple aperture structure per shaped like a cone or a pore (Figure 10B, C). The radial distribution of pseudopodia issuing from live specimens suggested they may be arising from more than one aperture (Figure 10D).
Comments
The most distinctive feature of the Agglutinated sphere described here is the presence of magnetic particles and other mineral particles in the cytoplasm, as with the Psammophaga sp. described here. All the known Psammophaga species, however, are oval with a single unmistakable terminal aperture structure. The apertures on raised papillae seen in the Agglutinated spheres are reminiscent of the genus Thurammina Brady, Reference Brady1879 although the test walls in the Thurammina species are more solid and rigid and the papillae are more prominent (Heron-Allen & Earland, Reference Heron-Allen and Earland1917). There is no evidence as of yet that the apertures of the Agglutinated sphere are distributed evenly around the test as in Thurammina, and this will require further future investigation.
A collection of what were described as being species of the genus Psammosphaera Schulze, 1875, and collected from the Adriatic Sea (Sabbatini et al., Reference Sabbatini, Bonatto, Gooday, Morigi, Pancotti, Pucci and Negri2010, plate 3) were described as having a spherical test, an organic wall with attached grains and a granular cytoplasm and one of the species possessed a set of dark inclusions. These tests of these specimens did not appear to have any visible apertures and they were much smaller than the Agglutinated sphere, with test sizes ranging from 125–150 μm, and previous reports have described Psammosphaera as lacking in obvious aperture structures (Gooday, Reference Gooday2002). A more likely counterpart to the Agglutinated sphere is Allogromiid species 8 from Tempelfjord, Svalbard (Sabbatini et al., Reference Sabbatini, Morigi, Negri and Gooday2007, plate I/L), described as being spherical to sub-spherical in shape with a small, puckered, indistinct aperture and a fine-grained cytoplasm with numerous small inclusions, but it was much smaller than the Agglutinated sphere, with a typical size of 126 μm. In some of the other allogromiid-like species in the Svalbard collection, the organic theca was enclosed within an agglutinated casing or what could be described as a ‘secondary test’.
Another candidate from the bathyal and abyssal Weddell Sea that might be related to the Agglutinated sphere is Bathyallogromia weddellensis Gooday et al., Reference Gooday, Holzmann, Guiard, Cornelius and Pawlowski2004 (Gooday et al., Reference Gooday, Holzmann, Guiard, Cornelius and Pawlowski2004, figures 2, 4 and 5), described as being light in colour, approximately spherical in shape, 251 μm long and 231 μm wide, with a colourless test wall and a cytoplasm that is finely granular and with numerous mineral inclusions scattered within. As well as being smaller than the Agglutinated sphere, however, B. weddellensis has a more pronounced aperture structure and no outer agglutinated layer to the test wall, and some individuals possess peduncular sheaths.
Agglutinated dome 1
Specimens were collected from five sites, and were found attached to various substrates including seaweed, pebbles or mollusc shells. Upon detachment from their substrates the majority remained alive and exhibited pseudopodia typical of foraminifera (Figures 11A, D and 12C, D). The dimensions for the collected specimens (N = 47) are length: 475 ± 197 μm, width: 362 ± 163 μm and length/width: 1.4 ± 0.3 indicating a largely circular to oval profile as seen from above. The common feature of these specimens is an agglutinated test wall of a matt white texture in which are embedded minute, colourless mineral grains (Figures 11, 12). The test is either surrounded by a ring of sediment, or patches of sediment. The shape of the agglutinated dome varies from being hemispherical (Figure 11A, C), half oval (Figures 11B, D, 12A–C) or with an irregular rim (Figure 12A, D). The shape is often determined by the substrate, whether shell (Figure 11A, B), stone (Figure 11C, D) or seaweed (Figure 12). When released from the substrate and turned over, the colour of the cytoplasm is seen to range from colourless to deep orange. It could not be determined whether the choice of substrate and colour of cytoplasm represented different species as there was no clear morphological demarcation between the specimens at the light microscopic level.

Fig. 11. Agglutinated dome 1 with mollusc shell as substrate: (A & B) top and bottom view of round and oval specimens, and pseudopodia; Agglutinated dome 1 with rock as substrate: (C & D) top and bottom view of round and oval specimens, and pseudopodia.

Fig. 12. Agglutinated dome 1 with seaweed as substrate: (A) collection of specimens attached to red seaweed; (B) top (attached) and bottom (detached) view of oval specimen; (C) oval specimen (detached) with pseudopodia; (D) top (attached) and bottom (detached) view of irregular-shaped specimen, and its pseudopodia.
Comments
The closest counterpart to the Agglutinated dome 1 is Tholosina protea Heron-Allen & Earland, 1932a (Heron-Allen & Earland, Reference Heron-Allen and Earland1932a, plate 8/5−8) that was common in dredgings from around the British coastline, and illustrated by Rhumbler (Reference Rhumbler1935, plate 5/70–72). It was described by Heron-Allen & Earland as having ‘a thick-walled test agglutinated with very fine mineral grains, the colour varying from snowy white, the most common, to nearly black, according to the material employed; there are one or more small apertures situated at the extremities and the “protean” shape of the test is dependent by the nature of the surface of attachment: on flat surfaces it is semi-globular, sometimes nearly globular, but when attached to the stalk of a zoophyte it takes on many shapes and a favourite position is at the forking angle of a zoophyte where it becomes attached to both branches, or it surrounds a slender branch’. Tholosina protea is of comparable size to that of the Agglutinated Dome 1, and was regarded by Heron-Allen & Earland (Reference Heron-Allen and Earland1932a) as being the shallow-water equivalent of the deep-sea type species Tholosina bulla (Brady, Reference Brady1881), originally called Placopsilina bulla (Brady, Reference Brady1881) and illustrated by Brady (Reference Brady1884). Other agglutinated domes of these descriptions, attached to shells, were illustrated by Voltski & Pawlowski (Reference Voltski and Pawlowski2015, plate 1/C, I) and were designated as type taxon Flexammina islandica Voltski & Pawlowski, Reference Voltski and Pawlowski2015.
Agglutinated dome 1 is also similar looking to Crithionina granum Goës, Reference Goës1894, type taxon Crithionina mamilla Goës, Reference Goës1894 from the North Sea, designated and illustrated by Goës (Reference Goës1894). Crithionina mamilla was also found in large numbers in the waters of western Scotland by Heron-Allen & Earland (Reference Heron-Allen and Earland1916), who described it as having a thick-walled test agglutinated with very fine mineral particles, and in some regions, agglutinated with sponge spicules and shell fragments. Another Crithiona type from the North Sea, described as being chalky white, is Pseudowebbinella goesi (Höglund, Reference Höglund1947), now going by the name of Crithionina goesi Höglund, Reference Höglund1947. The illustration of Crithionina goesi by Höglund (Reference Höglund1947) shows a test with a thin base on the side of the test that is adherent to the substrate. Thus it is not unlikely that other species of the agglutinated dome type also possess a thin base to the test that becomes destroyed or left behind when the specimen is detached from its substrate.
Agglutinated dome 2
Seven specimens of this large variety, attached to seaweed or pebbles, were collected from three sites. The dimensions of the collected specimens (N = 7) are length: 996 ± 287 μm, width: 671 ± 240 μm and length/width: 1.5 ± 0.2. The Agglutinated dome 2 is stellate-shaped when attached to its substrate and has an agglutinated test wall with mineral granules of various sizes, larger than for the Agglutinated dome 1, mostly colourless, but some of the mineral granules are pigmented. The tests in their natural state are well-camouflaged by a covering of sediment and can be mistaken for lumps of debris. There is no apparent difference in morphology between those attached to seaweed (Figure 13A) and stone (Figure 13B). When a specimen is detached and turned over, this reveals a clump of bright orange-yellow cytoplasm inside the test, looking like egg yolk (Figure 13A, B). It is probable that a base layer is destroyed upon detachment of the test in this species also. The cells exhibit the pseudopodia of the foraminifera type (Figure 13A2).

Fig. 13. Agglutinated dome 2 with seaweed as substrate: (A) top (attached) and bottom (detached) view of specimen, showing coarse mineral granules on the test wall, and pseudopodia; Agglutinated dome 2 with rock as substrate: (B) top (attached) and top (detached) view; the orange-coloured cytoplasm is visible through the test wall.
Comments
The species is similar to or probably corresponds to type species Iridia diaphana Heron-Allen & Earland, Reference Heron-Allen and Earland1914. This was first found in the Indian Ocean attached to stone and shell (Heron-Allen & Earland, Reference Heron-Allen and Earland1914, plate 36) and is also illustrated by Rhumbler (Reference Rhumbler1935, plate 6/82–85). It is described as having a test with a single cavity lined with an organic wall to which is attached quite coarse sand grains or shell fragments, an organic floor to the test, what may be an aperture at the side or top of the dome, or no visible aperture, and irregular in outline, owing to a haphazard mode of growth. The species was later recorded by Heron-Allen & Earland to be found in British waters, of a much smaller size than the tropical version which can reach up to 8 mm in diameter. Iridia cf. I. diaphana, is also a common monothalamid found attached to a variety of seagrass and algal substrates in Florida Bay (Habura et al., Reference Habura, Goldstein, Broderick and Bowser2008, figure 1D).
Discussion
Novelty and limitations of the study
This is the first report on the diversity of soft-walled monothalamids in an intertidal habitat in north-west Scotland, a temperate part of the north-eastern Atlantic shoreline. The places favoured by these monothalamids is the muddy sediment, revealed at very low tide, of sheltered estuaries and bays fed by rivers with shallow inclines and minimal wave action. These advantageous sites are typically richly populated also by other fauna such as burrowing worms, a great variety of meiofauna, hard-shelled foraminifera and Gromia oviformis. The monothalamids described here most likely represent novel species and only two could be assigned to genus level, namely the Vellaria sp. and the Psammophaga sp. A previous molecular study on samples from deeper water in the Dunstaffnage region identified some species of Psammophaga (Pawlowski et al., Reference Pawlowski, Majewski, Longet, Guiard, Cedhagen, Gooday, Korsun, Habura and Bowser2008) and these could be the same, or close relatives of the Psammophaga sp. decribed here.
Only one other study has been done on monothalamids in a British intertidal region, in an estuary on the south coast of England, where an abundance of Psammophaga species was found (Larkin & Gooday, Reference Larkin and Gooday2004). Other intertidal monothalamids have been described in much warmer, tropical or subtropical areas, namely the Vellaria genus which was discovered in an estuary in India (Gooday & Fernando, Reference Gooday and Fernando1992), Iridia diaphana in a sub-tropical reef system in Florida Key, and various species of Allogromia and Psammophaga monothalamids in low salinity salt marshes at Sapelo Island Georgia (Altin et al., Reference Altin, Habura and Goldstein2009; Goldstein et al., Reference Goldstein, Habura, Richardson and Bowser2010; Habura et al., 2010; Altin-Ballero et al., Reference Altin-Ballero, Habura and Goldstein2013).
More numerous investigations have probed the monothalamids in shallow (<10 m) waters in the Black Sea (Gooday et al., Reference Gooday, Anikeeva and Sergeeva2006, Reference Gooday, Anikeeva and Pawlowski2010; Anikeeva et al., Reference Anikeeva, Sergeeva and Gooday2013) and the Adriatic Sea (Sabbatini et al., Reference Sabbatini, Bonatto, Gooday, Morigi, Pancotti, Pucci and Negri2010, Reference Sabbatini, Nardelli, Morigi and Negri2013). These two seas are much warmer than around the coast of Scotland and both have very narrow connections to the open ocean, habitats which may have encouraged the great diversity of monothalamid species described from there, including undetermined versions of Shepheardellinae, and Psammophaga, and many unknown saccamminids and allogromiids of various shapes, some of which also may have included novel species of Gromia. A marine environment more similar to that of the coast of west Scotland, but a few degrees colder, is the Icelandic coast where sampling was done from a sandy bay next to Vogar, south-west Iceland (Voltski & Pawlowski, Reference Voltski and Pawlowski2015); these samples contained a number of species similar to those found in the Scotland batches.
The two main drawbacks of the current work are the lack of quantitative and molecular data on the organisms found on the beach in north-west Scotland. Since no intertidal studies had been done before on this area, it was important in the first instance to report on where the species can be found and at what time of year, in order to build up a large enough portfolio of morphotypes to make further studies on them worthwhile. One difficulty of working with intertidal monothalamid species is not just that they only favour limited types of habitats and only at certain times of the year, but also the covert existence of some of the inhabitants which are either very small, hidden in mud balls or, with respect to the attached variety, are concealed by liberal amounts of sediment. Finding the optimal seasons and habitats for collecting a good diversity of samples required some initial sampling done in many different types of intertidal coastal environments, at different tidal levels along the beach and at various times of the year. Sieving with fine meshes was not carried out so as not to overlook smaller specimens, which were only seen by coverslip-capture, and not risk damage to larger, delicate specimens such as those surrounded by mud balls. It is possible, however, that some of the smaller types of monothalamids were merely juvenile versions of larger, sometimes unseen specimens, as size is usually an indication of age (Murray & Alve, Reference Murray and Alve2000; Altin et al., Reference Altin, Habura and Goldstein2009; Goldstein & Alve, Reference Goldstein and Alve2011; Altin-Ballero et al., Reference Altin-Ballero, Habura and Goldstein2013). To determine whether this is the case will require culturing the specimens for extended periods of time because foraminifera have complex reproductive cycles, but this has proved to be difficult for most species of foraminifera (Goldstein, Reference Goldstein and Sen Gupta1999).
There are other problems in the interpretation of specimens found on intertidal zones. Some varieties, especially the elongated monothalamids that prefer to live on seaweed rather than in the sediment, were not commonly encountered at any time of the year. It cannot be discounted that their preferred habit is on seaweed in deeper photic zones, and that the specimens observed could have strayed, or got washed into an environment that was not entirely favourable to them. To determine whether this is the case will require parallel sampling from deeper waters in the vicinity of where the beach versions were found.
Every effort was made to preserve the natural colour of the specimens and viability and was determined by the presence of a granulo-reticulopodian network. Pseudopodia were extended within minutes of placing the specimen in a well slide and, although all exhibited the classical granulo-reticularpodia features, there was quite a variety in the architecture of the pseudopodia in different species: some of the pseudopodia enveloped the test wall, others had a rich network of processes and others were more sparsely endowed. The question therefore arises as to whether the granulo-reticulopodian network does indeed possess distinguishing characteristics for species identification, or whether the observed behaviour is of little consequence due to the unnatural circumstances in which they are being exhibited.
Secondary tests
An interesting feature of certain benthic monothalamid species is the way some surround their primary test with a looser external covering made up of assembled particles from the environment, cemented with some additional organic matter. This is quite common among multi-chambered foraminifera as well, and the construction is sometimes referred to as a ‘secondary test’ or as a feeding or a reproductive test. The secondary test is not as robust as the regular agglutinated test because the secondary test covering can be destroyed by rough sampling or by the sieving commonly used when processing smaples. There are different types of secondary test architecture and in some cases the secondary test structure extends out tubes that protect the pseudopodia (Cedhagen, Reference Cedhagen1996; Heinz et al., Reference Heinz, Geslin and Hemleben2005).
In the present study two forms of secondary test were observed. The first type, associated with Allogromiid sp. 1, is a loosely bound structure composed of two layers, an outer muddy layer that comes away easily and a tougher inner layer of more consolidated sediment. In some individuals the inner layer comes away cleanly to reveal a transparent test with a reflective (shiny) surface; in others, the inner cyst wall is more strongly adhered to the test wall. Mud balls made of glued sediment with a loose cavity containing a delicate small spherical allogromiid-like test have been described for specimens from Admiralty Bay, King George Island, West Antarctica (Majewski et al., Reference Majewski, Lecroq, Sinniger and Pawlowski2007) and from western Svalbard (Gooday et al., Reference Gooday, Bowser, Cedhagen, Cornelius, Hald and Korsun2005).
The second type of cyst construction is associated with what is referred to in the literature as the ‘encased allogromiid’, and the Agglutinated spheres fall into this category. Here the test wall appears to be agglutinated, but the agglutination is not strongly bound and patches of mineralisation shear off easily. There is often an apparent gradation in agglutination of different specimens, and beneath the loosely bound mineralized sheath is a transparent proteinaceous test wall. ‘Encased’ monothalamid species with walls of this nature are fairly common (Gooday et al., Reference Gooday, Bowser and Bernhard1996; Pawlowski et al., Reference Pawlowski, Fahrni, Guiard, Conlan, Hardecker, Habura and Bowser2005; Sabbatini et al., Reference Sabbatini, Morigi, Negri and Gooday2007, Reference Sabbatini, Bonatto, Gooday, Morigi, Pancotti, Pucci and Negri2010, Reference Sabbatini, Nardelli, Morigi and Negri2013).
Influence of the environment and seasons
Some of the monothalamid species described here could be found only in large numbers during certain months either in spring or autumn, and this applied to Psammophaga sp. and the Agglutinated spheres, as well as to some of the saccamminids and undetermined monothalamids. The summer months were the least favourable, especially as now the summers seem to getting warmer, because the substrates used by the monothalamids such as the surfaces of muddy sediments, seaweed, discarded mollusc shells and pebbles become covered by blooms of various types of algae. It would indeed be of interest to perform a more systematic study of the seasonal distribution of beach monothalamids, and also to determine how they are distributed in relation to the other types of foraminifera, to the meiofauna and to the Gromia found there.
The seasonal abundance of any monothalamid is a property likely to be missed in the ‘once only’ sampling from deep waters in remote places. On the other hand, the seasonal availability may be exclusive to those living in shallow water and especially intertidal ones. Unlike their deep-water relatives, intertidal foraminifera are more exposed to extreme changes in temperature, pH and salinity and other factors mediated by vagaries of the climate and seasonal change (Golikova et al., Reference Golikova, Varfolomeeva, Yakovis and Korsun2020; Weinmann et al., Reference Weinmann, Goldstein, Triantaphyllou and Langer2021). To survive they have to be especially adapted to live in the transitional environments of estuaries and other environments influenced by inputs of fresh water, organic matter and oxygen levels (Ellison, Reference Ellison1984; Gooday & Fernando, Reference Gooday and Fernando1992; Larkin & Gooday, Reference Larkin and Gooday2004; Gooday et al., Reference Gooday, Bowser, Cedhagen, Cornelius, Hald and Korsun2005; Sabbatini et al., Reference Sabbatini, Morigi, Negri and Gooday2007, Reference Sabbatini, Bonatto, Gooday, Morigi, Pancotti, Pucci and Negri2010, Reference Sabbatini, Nardelli, Morigi and Negri2013). Foraminifera in general possess a high metabolic rate and are able to respond rapidly to an input of organic matter caused by blooms in the overlying water column and by seasonal variability of available organic matter (Gooday et al., Reference Gooday, Levin, Linke, Heeger, Rowe and Pariente1992; Richardson & Cedhagen, Reference Richardson and Cedhagen2001; Sabbatini et al., Reference Sabbatini, Bonatto, Bianchelli, Pusceddu, Danovaro and Negri2012). Monothalamids were the earliest-evolving foraminifera (Pawlowski et al., Reference Pawlowski, Holzmann, Berney, Fahrni, Gooday, Cedhagen, Habura and Bowser2003), so they will have been prominent members of benthic communities for longer than the hard-shelled foraminifera and they would have had an important stabilising influence by feeding on bacteria and other micro-organisms and acting as prey for larger organisms (Goldstein, Reference Goldstein and Sen Gupta1999).
The intertidal foraminifera are also more likely than their deeper cousins to be exposed to coastal environmental pollution caused by human activity. The areas in which many of the samples were collected are fed by lochs that have fish farms, marinas and housing around them, so the specimens are likely to be exposed to organic enrichment related to the fish farming and urban activities. Members of the genus Psammophaga especially seem to prefer eutrophic conditions (Pawlowski & Majewski, Reference Pawlowski and Majewski2011; Sabbatini et al., Reference Sabbatini, Bonatto, Bianchelli, Pusceddu, Danovaro and Negri2012; Altin-Ballero et al., Reference Altin-Ballero, Habura and Goldstein2013) and are often found in sediment close to fish farms (Pawlowski et al., Reference Pawlowski, Esling, Lejzerowicz, Cedhagen and Wilding2014). Longitudinal studies on beach communities of monothalamid species may help to increase our understanding of how they deal with difficult and unstable environments and could provide early warning indicators of both climate change (Jonkers et al., Reference Jonkers, Hillebrand and Kucera2019; Fox et al., Reference Fox, Stukins, Hill and Miller2020) and burgeoning levels of local marine pollution (Martins et al., Reference Martins, Pinto, Frontalini, da Fonseca, Terroso, Laut, Zaaboub, da Conceição Rodrigues and Rocha2016; Frontalini et al., Reference Frontalini, Greco, Di Bella, Lejzerowicz, Reo, Caruso, Cosentino, Maccotta, Scopelliti, Nardelli, Losada, Armynot du Châtelet, Coccioni and Pawlowski2018; Brouillette Price et al., Reference Brouillette Price, Kabengi and Goldstein2019; Smith & Goldstein, Reference Smith and Goldstein2019).
Conclusions
• This is the first report on the rich assemblage of novel benthic monothalamid foraminifera, belonging to at least 13 morphotypes, found in intertidal zones in north west Scotland.
• The highest diversity of monothalamid species were located in sheltered muddy bays fed by rivers and in large estuaries bordering the mouth of major sea lochs.
• All of the monothalamids are undescribed species but most bear resemblance to, and are likely to be related to, monothalamid species previously reported to live in intertidal zones in other parts of the world and in shallow and deep waters around Scotland and in both warmer and cooler parts of the globe.
Data statement
All data underlying the results are available as part of the article and no additional source data are required.
Acknowledgements
I thank the two referees for their many helpful comments for improving the manuscript, Jeremy Poole for processing and supplying a scanning electron microscope of the specimen featured in Figure 5G, and the University of Leeds for providing me (as a retired Senior Research Fellow) with access to Library services. I acknowledge also the open access availability of the QGIS: GNU program and data files for reproducing maps by General public licence Version 2, June 1991 and of the mapping databases provided by the Diva GIS system (www.diva-gis.org) and OS OpenData: © Crown copyright Version: 2022-05, Open Government Licence: https://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.