INTRODUCTION
Marine caves are characterized by environmental gradients generating spatial variability of sessile colonizers (Harmelin, Reference Harmelin1985, Reference Harmelin1986; Bianchi & Morri, Reference Bianchi and Morri1994; Bussotti et al., Reference Bussotti, Terlizzi, Fraschetti, Belmonte and Boero2006). These complex habitats constitute ‘biodiversity reservoirs’ of high conservation value, harbouring endemic species and ‘ancient’ forms (Bianchi et al., Reference Bianchi, Morri, Chiantore, Montefalcone, Parravicini, Rovere and Stambler2012; Gerovasileiou & Voultsiadou, Reference Gerovasileiou and Voultsiadou2012). Although Mediterranean marine cave communities have been the subject of research in several studies, considerable knowledge gaps still exist, with regard to certain regions (e.g. eastern and southern Mediterranean sectors) and taxonomic groups (Gerovasileiou et al., Reference Gerovasileiou, Chintiroglou, Vafidis, Koutsoubas, Sini, Dailianis, Issaris, Akritopoulou, Dimarchopoulou and Voultsiadou2015, Reference Gerovasileiou, Martínez, Álvarez, Boxshall, Humphreys, Jaume, Becking, Muricy, van Hengstum, Dekeyzer, Decock, Vanhoorne, Vandepitte, Bailly and Iliffe2016a).
Serpulid polychaetes are among the dominant sessile taxa in confined marine cave systems (Zibrowius, Reference Zibrowius1968, Reference Zibrowius1971; Belloni & Bianchi, Reference Belloni and Bianchi1982; Bianchi, Reference Bianchi1985; Harmelin et al., Reference Harmelin, Vacelet and Vasseur1985; Bianchi & Sanfilippo, Reference Bianchi, Sanfilippo, Cicogna, Bianchi, Ferrari and Forti2003), sometimes forming bioconstructions that resemble pseudo-stalactites. Such formations have been observed to date in the Caribbean Sea (Macintyre et al., Reference Macintyre, Rützler, Norris and Fauchald1982), the Tyrrhenian Sea in the western Mediterranean (Antonioli et al., Reference Antonioli, Silenzi and Frisia2001) and the southern Adriatic and Ionian seas in the central Mediterranean (Belmonte et al., Reference Belmonte, Ingrosso, Poto, Quarta, D'Elia, Onorato and Calcagnile2009; Quarta et al., Reference Quarta, D'Elia, Calcagnile, Belmonte and Ingrosso2010; Sanfilippo et al., Reference Sanfilippo, Rosso, Guido, Mastandrea, Russo, Ryding and Taddei-Ruggiero2015).
The diversity of serpulids in marine cave habitats has been investigated mostly in the western Mediterranean Sea (e.g. Zibrowius, Reference Zibrowius1968; Di Geronimo et al., Reference Di Geronimo, LaPerna, Rosso and Sanfilippo1993; Taddei-Ruggiero et al., Reference Taddei-Ruggiero, Annunziata, Rosso and Sanfilippo1996; Bianchi & Sanfilippo, Reference Bianchi, Sanfilippo, Cicogna, Bianchi, Ferrari and Forti2003; Harmelin et al., Reference Harmelin, Boury-Esnault, Fichez, Vacelet and Zibrowius2003; Rosso et al., Reference Rosso, Sanfilippo, Taddei-Ruggiero and Di Martino2013). To date, a total of 51 serpulid species have been reported from Mediterranean marine caves in the literature (V. Gerovasileiou, unpublished data).
The research effort recently invested in marine caves of the eastern Mediterranean has raised the regional biodiversity known from this habitat type (Gerovasileiou et al., Reference Gerovasileiou, Chintiroglou, Vafidis, Koutsoubas, Sini, Dailianis, Issaris, Akritopoulou, Dimarchopoulou and Voultsiadou2015). Most studies, however, focused on other taxa, which dominate in marine caves, such as sponges (e.g. Gerovasileiou & Voultsiadou, Reference Gerovasileiou and Voultsiadou2016). Bailey (Reference Bailey1969) recorded eight Spirorbinae species from a small submerged cave in Chios Island (North Aegean Sea) but most serpulid species known from the Aegean ecoregion have been reported from habitats other than caves (Knight-Jones et al., Reference Knight-Jones, Knight-Jones and Ergen1991; Koçak et al., Reference Koçak, Ergen and Çinar1999; Arvanitidis, Reference Arvanitidis2000; Bianchi & Morri, Reference Bianchi and Morri2000; Çınar et al., Reference Çinar, Dağli and Kurt Şahin2014).
The aim of this paper was to (1) provide a first detailed record of the diversity of serpulids in submerged caves of the under studied eastern Mediterranean; (2) examine the structure of serpulid assemblages in this area, focusing on aspects of their morphology, ecology and growth form; and (3) investigate their distribution patterns along different cave sectors.
MATERIALS AND METHODS
Study areas
The surveyed caves are located off Lesvos Island (Aegean Sea, eastern Mediterranean) (Figure 1A). Agios Vasilios cave (38.969°N 26.541°E) is a blind cave located at a depth range of 24–40 m (Figure 1B). Fara cave (38.969°N 26.477°E) is 32 m long and has the shape of a tunnel, ending in a dark chamber connected to a second cave through a fissure (Figure 1B). The entrance of the cave is located at 18 m while the average depth inside the cave is 14 m. Both caves are formed in Triassic carbonate rocks (Katsikatsos et al., Reference Katsikatsos, Matarangas, Migiros and Triantaphyllis1982). The two caves were mapped and depicted in three-dimensional models with ‘cavetopo’ software (Gerovasileiou et al., Reference Gerovasileiou, Trygonis, Sini, Koutsoubas and Voultsiadou2013a). The structure of their sessile communities has been previously described (Gerovasileiou et al., Reference Gerovasileiou, Vafidis, Koutsoubas and Voultsiadou2013b, Reference Gerovasileiou, Koutsoubas, Voultsiadou, Langar, Bouafif and Ouerghi2014; Gerovasileiou & Voultsiadou, Reference Gerovasileiou and Voultsiadou2016) but their serpulid diversity has not been studied.

Fig. 1. Map of Lesvos Island in the Aegean ecoregion, showing the locations of the studied caves (A). Scaled three-dimensional depictions (lateral and plan views) of Fara and Agios Vasilios caves produced with ‘cavetopo' software (Gerovasileiou et al., Reference Gerovasileiou, Trygonis, Sini, Koutsoubas and Voultsiadou2013a) and location of sampling sites (B).
Sampling process and sample analysis
Three replicate quadrats of 400 cm2 (20 × 20 cm) were scraped at 10 sampling stations in the surveyed caves (six in Fara cave and four in Agios Vasilios cave) in 2010 by scuba diving using the quadrate sampler designed by Chintiroglou & Koukouras (Reference Chintiroglou and Koukouras1992). Sampling stations (Figure 1B, Table 1) represented different assemblages of the sidewalls and ceiling at different distances from the entrance of the caves. Photographs of serpulid bioconstructions were also taken in situ. Samples were sieved (0.5 mm) and preserved in 10% formalin. After the sorting process, serpulids were identified at the species level under a stereomicroscope.
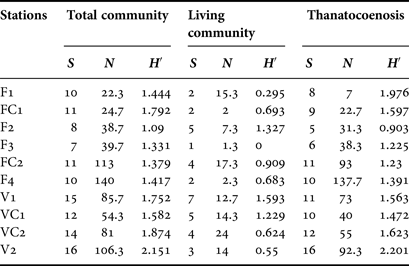
Table 1. Description of the sampling sites for all stations (i.e. distance from the entrance and position on the walls of the cave) and their basic community structure (F: Fara cave, V: Agios Vasilios cave).

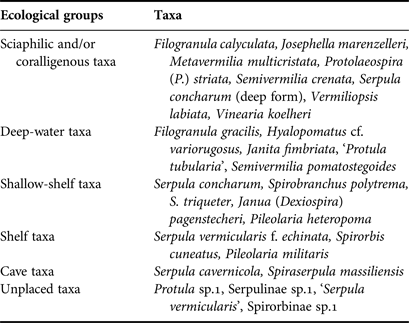
For each station, living communities and thanatocoenoses (empty tubes) were examined, and the serpulid abundance (N), number of taxa (S) and Shannon–Wiener diversity index (H′) were calculated. Moreover, all taxa were assigned to six ecological groups according to Rosso et al. (Reference Rosso, Sanfilippo, Taddei-Ruggiero and Di Martino2013 and references therein), specifically: (1) ‘Cave species’ including sciaphilic species which thrive in caves as well as in other cryptic micro-habitats (cavities or crevices); (2) ‘Sciaphilic and/or coralligenous species’ which show preference for shadowy and sheltered biotopes such as overhangs, coralligenous concretions, and the lower surface of small-sized hard substrata lying on soft bottoms; (3) ‘Deep-water species’, which are usually found on the outer shelf and upper slope (lower circalittoral and upper bathyal zones); (4) ‘Shelf species’, including more or less euryoecious species which are widely distributed in shelf environments; (5) ‘Shallow-shelf species’ including light-tolerant species able to colonize infralittoral to upper circalittoral habitats; (6) ‘Unplaced species’ which cannot be placed in any of the above groups. The latter group includes species for which no distributional preference is known and taxa identified at high taxonomic levels.
Low magnification images of selected specimens were taken with a Zeiss Discovery V8A stereomicroscope equipped with an Axiocam MRC and Axiovision acquisition system. Specimens are deposited in the Palaeontological Section of the Earth Science Museum at the University of Catania (Italy).
Statistical analysis
One-way permutational ANOVA (perANOVA) was used in order to investigate the variability of the above biotic measures across the stations of each cave (factor, Station; fixed with six levels for Fara cave and four levels for Agios Vasilios cave). Resemblance of the serpulid community in the different sampling stations of the caves was examined with multidimensional scaling (MDS), based on the Bray–Curtis similarity index (fourth root transformed mean abundance data).
The contribution of serpulid taxa to the similarity of samples within the resulting groups was estimated with SIMPER (SIMilarity PERcentages). Statistical analysis was undertaken using PRIMER-E v6 software package (Clarke & Gorley, Reference Clarke and Gorley2006).
RESULTS
Taxonomic composition
A total of 27 serpulid taxa were identified in the two caves (19 Serpulinae and 8 Spirorbinae), 21 of which were common in both caves (16 Serpulinae and 5 Spirorbinae). Living serpulid assemblages consisted of 14 taxa (10 Serpulinae and 4 Spirorbinae), 6 of which (5 Serpulinae and 1 Spirorbinae) were found in both caves (Table 2). The thanatocoenoses consisted of 26 taxa (19 Serpulinae and 7 Spirorbinae), of which 18 were common in the two caves (15 Serpulinae and 3 Spirorbinae). The most widely distributed and abundant species were Semivermilia crenata, Josephella marenzelleri and Metavermilia multicristata, in all cases. The remaining taxa presented much lower abundances. Selected photographs of serpulid specimens are presented in Figure 2.

Fig. 2. Photographs of serpulid specimens from different stations of the studied marine caves (in parentheses): (A) Serpula cavernicola (V1); (B) ‘Serpula vermicularis’ (Fara cave); (C) Serpula vermicularis f. echinata (V1); (D) Serpula concharum (FC2); (E) Spiraserpula massiliensis (VC2); (F) Vermiliopsis labiata (V1); (G) Metavermilia multicristata (F4); (H) Semivermilia crenata (V2); (I) Spirobranchus polytrema (FC1); (J) Hyalopomatus cf. variorugosus (V2); (K) Josephella marenzelleri (V1); (L) ‘Protula tubularia’ (F4); (M) Pileolaria heteropoma (FC1); (N) Vinearia koehleri (V1). Scale bars: 1 mm for A–K and M–N, 1 cm for L. Photos by R. Sanfilippo (A, C–K, M–N), T. Dailianis (B) and V. Gerovasileiou (L).
Table 2. Systematic list of serpulid taxa recorded in the studied Aegean caves (bold font indicates a living community).

Spatial patterns
Serpulid abundance increased inwards in both caves (Table 3); however, this variability was significant only in Fara cave, for the living community (df = 5, Pseudo-F = 3.5903, P < 0.05), the thanatocoenosis (df = 5, Pseudo-F = 10.185, P < 0.01), and the total community (df = 5, Pseudo-F = 8.2601, P < 0.01). The number of taxa and the Shannon–Wiener diversity index did not change significantly in either of the two caves. Higher numbers of taxa were recorded in all stations of Agios Vasilios cave compared with those of Fara cave (Table 3).
Table 3. Number of taxa (S), mean abundance (N) and Shannon–Wiener diversity (H′) per sampling station for the total and living communities and for the thanatocoenoses, respectively.
Multivariate resemblance analysis revealed three major groups of stations with similarity greater than 60%, as shown in the MDS plot (Figure 3): (1) stations from the semi-dark sector of Fara cave (F3 with FC1); (2) stations from the semi-dark sector of Agios Vasilios cave (VC1 with V1); and (3) stations from the dark sectors of both caves (FC2, F4, VC2 and V2), regardless of the position on the cave walls. The two outermost stations of Fara cave, F1 and F2, differed from the above groups. According to the results of SIMPER analysis, the taxa which contributed by 60% to the similarity of samples within the above groups were respectively (in order of appearance): (1) S. crenata, J. marenzelleri and Spirobranchus polytrema; (2) S. crenata, J. marenzelleri, Protolaeospira (P.) striata and Serpula vermicularis f. echinata; (3) S. crenata, J. marenzelleri, M. multicristata and ‘Protula tubularia’. On the other hand, the taxa which contributed to 60% of the dissimilarity between the above groups could be categorized as follows: (a) taxa with higher abundance in the inner dark cave sectors (e.g. Vermiliopsis labiata, ‘P. tubularia’, Spiraserpula massiliensis and M. multicristata); (b) taxa recorded exclusively in the dark cave sectors (Semivermilia pomatostegoides); (c) taxa with higher abundance in the semi-dark cave sectors (e.g. P. striata).
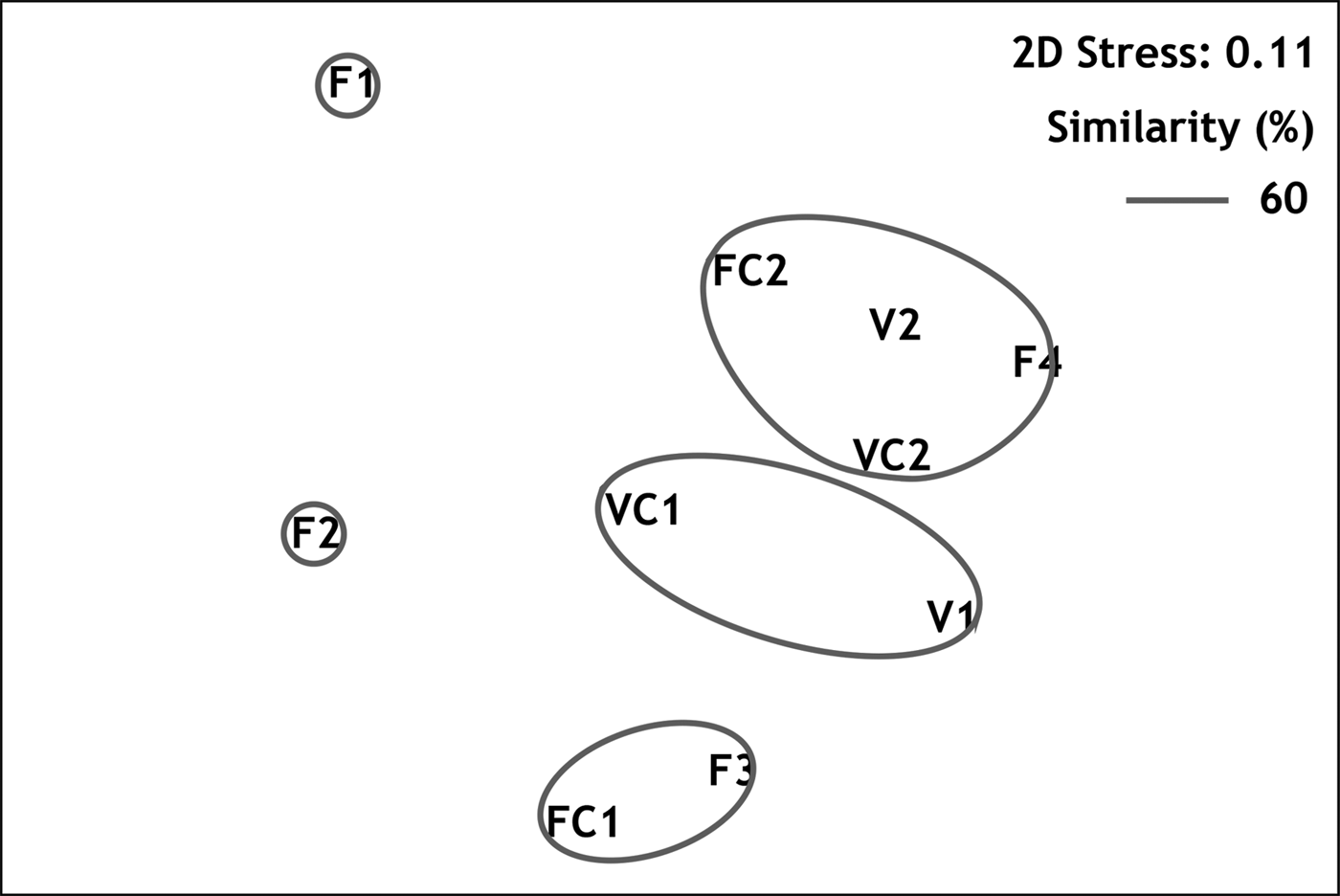
Fig. 3. Resemblance of sampling stations demonstrated in MDS plot.
Ecological groups
Sciaphilic/coralligenous serpulids prevailed in terms of number of taxa (8) in both caves, followed by deep-water (8), shallow-shelf (5), shelf (3), cave (2) and unplaced taxa (Table 4). The same order was recorded with regards to abundance. The abundance of the three first groups increased towards the dark interior of both caves (Figure 4). However, variability was significant only in Fara cave, for the sciaphilic/coralligenous (df = 5, Pseudo-F = 9.0973, P < 0.01) and deep-water groups (df = 5, Pseudo-F = 3.0735, P < 0.01).

Fig. 4. Contribution of the different ecological groups to the abundance of serpulids in the sampling stations of the studied caves.
Table 4. Assignment of the serpulid taxa recorded in the studied caves to ecological groups according to the classification scheme by Rosso et al. (Reference Rosso, Sanfilippo, Taddei-Ruggiero and Di Martino2013) and newly acquired data.
Bioconstructions and aggregates
Serpulids were more abundant in the innermost dark sectors where the rock was only partially covered by serpulids and coated by diffuse black oxide crusts (Figure 5A). They were mostly found as isolated tubes, but in the innermost sectors of the caves some taxa aggregated to form three-dimensional structures. ‘Giant’ isolated tubes (up to 1 m long) possibly belonging to the genus Protula (observed in situ) were among the largest encrusters in the inner dark edge of Agios Vasilios cave. Some ‘P. tubularia’ specimens were locally aligned (Figure 5B), or aggregated, forming bioconstructions, often associated with nodular bryozoan concretions (Figure 5C). Thick crusts of tubes, resulting from the superimposition of successive generations of different serpulid taxa, were obvious at places (Figure 5D).

Fig. 5. (A) Wall of Fara cave from the dark zone, partially covered by different species of serpulids and coated by diffuse black oxide crusts (20 × 20 cm sampled surface F4, 30 m from the entrance); (B) Aligned biostalactites along a fissure of the right wall in Agios Vasilios cave (20 m from the entrance); (C) Serpulid bioconstructions associated with nodular bryozoan concretions in the dark zone of Fara cave (25 m from the entrance); (D) Thick crusts of superimposed tubes formed by subsequent serpulid generations in Agios Vasilios cave (20 m from the entrance). Scale bars: 5 cm for B, 1 cm for C, D. Photos by V. Gerovasileiou.
Bioconstructions of ‘P. tubularia’ had two forms: (1) coiled ‘doughnuts’ of tubes (Figure 6A), up to 6 cm in diameter, resulting from the superimposition of tube whorls strictly adhering to each other (Figure 6B), sometimes presenting straight tube end (the diameter of tube whorls was often progressively increasing); and (2) plait-like aggregates, ~8 cm high and 4 cm wide, formed by irregularly coiled and intermingled tubes (Figure 6C–E). The doughnut-like formations were larger and more frequent inwards (Figure 6F). The plait-like structures were hanging from the ceilings and projected obliquely from the walls in inner semi-dark and dark cave sectors (Figure 6G).

Fig. 6. (A) Coiled ‘doughnuts’ of tubes belonging to the large-sized serpulid ‘Protula tubularia’ (15 m from the entrance, Agios Vasilios cave); (B) A doughnut-like Protula bioconstruction, showing several tube whorls of increasing diameter (5 m from the entrance, Agios Vasilios cave); (C) Biostalactites built by aggregated individuals of the serpulid ‘P. tubularia’ (25 m from the entrance, Fara cave); (D, E) Subconical biostalactite formed by aggregated Protula tubes and cemented micrite: cross section and longitudinal view (32 m from the entrance, Fara cave); (F) Wall in the dark zone of Fara cave, covered with doughnut-like formations (30 m from the entrance); (G) Cave wall with densely spaced biostalactites (~10 formations /cm2), obliquely projecting towards the center of Fara cave (20–30 m from the entrance); (H) Intricate mm-thick tube nets formed by the microserpulid Josephella marenzelleri associated with bryozoans, developing on a doughnut-like tube of Protula (sample V2, Agios Vasilios cave); (K) Detail of H showing locally recrystallized micrite sediments between tubes. Scale bars: 1 cm for figures A–G, 5 mm for H, 1 mm for K. Photos by V. Gerovasileiou (A and F), R. Sanfilippo (B, D–E, H–K) and M. Sini (C and G).
The microserpulid J. marenzelleri locally formed mm-thick crusts of intermingled tubes, resulting in three-dimensional intricate nets. They occurred in samples from the semi-dark and dark cave communities, coating the surface of bryozoan concretions and Protula tubes, and filling small cavities (Figure 6H). These Josephella ‘nets’ were locally recrystallized, with secondary large crystals substituting the tube walls and filling spaces between tubes (Figure 6K).
DISCUSSION
The results of the present study showed that the surveyed Aegean marine caves host a rich serpulid fauna (14 living and 27 dead taxa), comparable to that from marine caves in the western and central Mediterranean Sea, which had been thoroughly studied for their serpulid diversity; notable examples include: Bagaud cave (19 living taxa), in South France (Harmelin et al., Reference Harmelin, Boury-Esnault, Fichez, Vacelet and Zibrowius2003); Mitigliano (27 living taxa) and the semi-submerged Accademia (2 living and 20 dead taxa) caves in the Tyrrhenian Sea (Belloni & Bianchi, Reference Belloni and Bianchi1982; Bianchi, Reference Bianchi1985; Balduzzi et al., Reference Balduzzi, Bianchi, Boero, Cattaneo-Vietti, Pansini and Sarà1989; Di Geronimo et al., Reference Di Geronimo, LaPerna, Rosso and Sanfilippo1993; Rosso et al., Reference Rosso, Sanfilippo, Taddei-Ruggiero and Di Martino2013); Mazzere, Gymnasium, and Granchi caves (13 living and 32 dead taxa) in the Ionian Sea, Sicily (Rosso et al., Reference Rosso, Sanfilippo, Taddei-Ruggiero and Di Martino2013); the semi-submerged Ciolo cave (20 living) in the Ionian Sea, southern Apulia (Denitto & Licciano, Reference Denitto and Licciano2006).
The species Hyalopomatus cf. variorugosus is reported for the first time from the marine cave habitat. This species had been reported from bathyal bottoms of other Mediterranean regions, with living and dead specimens (Ben-Eliahu & Fiege, Reference Ben-Eliahu and Fiege1996; Sanfilippo, Reference Sanfilippo1998, Reference Sanfilippo2009; Rosso et al., Reference Rosso, Vertino, Di Geronimo, Sanfilippo, Sciuto, Di Geronimo, Violanti, Corselli, Taviani, Mastrototaro and Tursi2010), and from Pleistocene bathyal deposits of southern Italy (Di Geronimo et al., Reference Di Geronimo, D'Atri, La Perna, Rosso, Sanfilippo and Violanti1997). The remaining taxa have already been reported from other marine caves in the Mediterranean Sea (Di Geronimo et al., Reference Di Geronimo, LaPerna, Rosso and Sanfilippo1993; Taddei-Ruggiero et al., Reference Taddei-Ruggiero, Annunziata, Rosso and Sanfilippo1996; Bianchi & Sanfilippo, Reference Bianchi, Sanfilippo, Cicogna, Bianchi, Ferrari and Forti2003; Rosso et al., Reference Rosso, Sanfilippo, Taddei-Ruggiero and Di Martino2013). Serpulid communities from caves located at comparable depths along the Tyrrhenian and Ionian coasts of Sicily had a similar structure, with the species Semivermilia crenata and Josephella marenzelleri dominating (Rosso et al., Reference Rosso, Sanfilippo, Taddei-Ruggiero and Di Martino2013). Interestingly, some taxa (i.e. Serpula vermicularis f. echinata, Filogranula calyculata, H. cf. variorugosus, Serpulinae sp.1 and Spirorbinae sp.1) found in the studied Aegean caves were absent from marine caves of Sicily. On the other hand, several species that are known to be typical cave-dwellers (Rosso et al., Reference Rosso, Sanfilippo, Taddei-Ruggiero and Di Martino2013 and references therein) e.g. Filogranula annulata, Placostegus tridentatus, P. crystallinus, Semivermilia cribrata, Simplaria pseudomilitaris, Spirorbis marioni and Vermiliopsis monodiscus, were not found among the scraped samples. These differences can be attributed to the high biogeographic heterogeneity of sessile taxa in Mediterranean marine caves (Gerovasileiou & Voultsiadou, Reference Gerovasileiou and Voultsiadou2012) coupled with the locally patchy distribution of taxa inside caves (Harmelin, Reference Harmelin1986, Reference Harmelin1997; Rosso et al., Reference Rosso, Sanfilippo, Taddei-Ruggiero and Di Martino2013) or mere sampling biases. For instance, the species P. tridentatus and V. monodiscus were recently recorded as infauna in the canals of the sponges Agelas oroides and Aplysina aerophoba in the studied Aegean caves (Gerovasileiou et al., Reference Gerovasileiou, Chintiroglou, Konstantinou and Voultsiadou2016b). The majority of taxa recorded in this study (18 taxa, 67%), are also new elements for the marine cave fauna of the Aegean Sea, according to the most recent biodiversity census in this habitat type (Gerovasileiou et al., Reference Gerovasileiou, Chintiroglou, Vafidis, Koutsoubas, Sini, Dailianis, Issaris, Akritopoulou, Dimarchopoulou and Voultsiadou2015).
Two of the species identified in this study, H. cf. variorugosus and Serpula cavernicola are new records for the serpulid fauna of the Aegean ecoregion (Arvanitidis, Reference Arvanitidis2000; Ben Eliahu & ten Hove, Reference Ben-Eliahu and ten Hove2011; Çinar et al., Reference Çinar, Dağli and Kurt Şahin2014). The latter species is a typical cave-dweller, restricted to dark sectors of marine caves (Sanfilippo & Mòllica, Reference Sanfilippo and Mòllica2000). It has been rarely reported from the Ionian and Tyrrhenian seas, the Straits of Gibraltar and the southern coasts of Portugal in the Atlantic Ocean, usually found as empty tubes (Fassari & Mòllica, Reference Fassari and Mòllica1991; Taddei-Ruggiero et al., Reference Taddei-Ruggiero, Annunziata, Rosso and Sanfilippo1996; Sanfilippo & Mòllica, Reference Sanfilippo and Mòllica2000; Rosso et al., Reference Rosso, Sanfilippo, Taddei-Ruggiero and Di Martino2013). In the studied caves, only dead specimens of S. cavernicola were found in several stations (Table 1).
It has been suggested that marine caves function as ‘islands’, supporting isolated populations of sessile taxa (Muricy et al., Reference Muricy, Solé-Cava, Thorpe and Boury-Esnault1996), which often exhibit morphological adaptations to the local environmental conditions (Harmelin et al., Reference Harmelin, Vacelet and Vasseur1985). Furthermore, several cave-exclusive species have been recorded from only one or very few caves (Gerovasileiou & Voultsiadou, Reference Gerovasileiou and Voultsiadou2012; Gerovasileiou et al., Reference Gerovasileiou, Martínez, Álvarez, Boxshall, Humphreys, Jaume, Becking, Muricy, van Hengstum, Dekeyzer, Decock, Vanhoorne, Vandepitte, Bailly and Iliffe2016a). In this respect, a number of serpulid taxa recorded in the studied Aegean caves deserve further study from the taxonomic point of view (morphological and molecular). These include the serpulids identified only at higher-than-species taxonomic levels (i.e. Protula sp.1, Serpulinae sp.1, Spirorbinae sp.1) as well as the species ‘P. tubularia’ and ‘S. vermicularis’, whose cosmopolitan distribution has been questioned (Kupriyanova & Jirkov, Reference Kupriyanova and Jirkov1997; ten Hove & Kupriyanova, Reference ten Hove and Kupriyanova2009). Distinct forms of certain taxa (i.e. S. vermicularis f. echinata and S. concharum (deep form)), which belong to different ecological groups (Table 4), were found in the deeper Agios Vasilios cave. Targeted study of the above-mentioned taxa would also be interesting from an ecological and taxonomic point of view.
The results of the multivariate resemblance analysis, coupled with those of the distribution of the different ecological groups across the studied caves, revealed that the structure of the serpulid community in the inner zone differs from that of the more heterogeneous outer semi-dark sectors. Specifically, the abundance of sciaphilic, deep-water and cave taxa increased inwards, following the environmental gradients such as the loss of light and the increasing water confinement (Balduzzi et al., Reference Balduzzi, Bianchi, Boero, Cattaneo-Vietti, Pansini and Sarà1989; Bianchi & Morri, Reference Bianchi and Morri1994; Sanfilippo et al., Reference Sanfilippo, Rosso, Guido, Mastandrea, Russo, Ryding and Taddei-Ruggiero2015). This variability was more conspicuous in Fara cave and is possibly related to the higher level of confinement in this cave, as it is characterized by a more elongated shape and a narrower cross-sectional area, compared with the wider and less confined Agios Vasilios cave (Gerovasileiou et al., Reference Gerovasileiou, Trygonis, Sini, Koutsoubas and Voultsiadou2013a; Gerovasileiou & Voultsiadou, Reference Gerovasileiou and Voultsiadou2016). The considerably higher percentage of deep-water taxa in the inner dark cave sectors is in accordance with the hypothesis that dark caves form ‘deep-sea mesocosms’ in the shallow littoral zone, presenting faunal affinities to the deep-sea (Zibrowius, Reference Zibrowius1971; Vacelet et al., Reference Vacelet, Boury-Esnault and Harmelin1994; Harmelin & Vacelet, Reference Harmelin and Vacelet1997; Martínez et al., Reference Martínez, Di Domenico and Worsaae2013).
Serpulid bioconstructions in the form of ‘coiled doughnuts’ by ‘P. tubularia’ seem to be unknown from other Mediterranean caves and deserve to be further investigated. On the other hand, the plait-like aggregates are similar to biostalactites currently being studied in a cave in Cyprus (Guido et al., unpublished data) and to those found in other caves at comparable depths in the Apulian (Onorato et al., Reference Onorato, Forti, Belmonte, Costantini and Poto2003; Belmonte et al., Reference Belmonte, Ingrosso, Poto, Quarta, D'Elia, Onorato and Calcagnile2009) and Sicilian coasts of Italy, central Mediterranean (Guido et al., Reference Guido, Heindel, Birgel, Rosso, Mastandrea, Sanfilippo, Russo and Peckmann2013, Reference Guido, Rosso, Sanfilippo, Russo and Mastandrea2016a; Sanfilippo et al., Reference Sanfilippo, Rosso, Guido, Mastandrea, Russo, Ryding and Taddei-Ruggiero2015). These biostalactites consist of skeletonized invertebrates and syndepositional-cemented micrite deposited in situ through microbial metabolic activity (Guido et al., Reference Guido, Mastandrea, Rosso, Sanfilippo and Russo2012, Reference Guido, Heindel, Birgel, Rosso, Mastandrea, Sanfilippo, Russo and Peckmann2013, Reference Guido, Mastandrea, Rosso, Sanfilippo, Tosti, Riding and Russo2014, Reference Guido, Rosso, Sanfilippo, Russo and Mastandrea2016b). The aggregation of Protula specimens forming this type of biostalactite seems to be influenced by the local salinity gradients induced by the dilution of continental water inflows through fractures in the surrounding rocks (Guido et al., Reference Guido, Mastandrea, Rosso, Sanfilippo, Tosti, Riding and Russo2014; Sanfilippo et al., Reference Sanfilippo, Rosso, Guido, Mastandrea, Russo, Ryding and Taddei-Ruggiero2015). A similar process could be responsible for the formation of the numerous biostalactites aligned along fissures of the Agios Vasilios cave.
In conclusion, the present study provided a first record of the marine cave serpulid fauna in the Aegean Sea identifying basic community patterns and interesting faunal affinities with other habitats as well as new faunal element sand forms of bioconstructions for the Mediterranean marine cave habitat. The results of the present study suggest that several aspects of the marine cave communities are still poorly known and deserve to be investigated.
ACKNOWLEDGEMENTS
The authors would like to thank Eleni Voultsiadou, Alejandro Martínez and an anonymous reviewer for their constructive comments, Maria Sini and Thanos Dailianis for providing underwater photographs and Dimitris Poursanidis for designing the map of the study area.
FINANCIAL SUPPORT
This study was supported by the Research Funding Programme ‘Heracleitus II: Investing in knowledge society’ (EU and Greek national funds). VG also benefited from ‘Alexander S. Onassis Public Benefit Foundation’ fellowship for postgraduate studies. This is contribution number 423 of the Catania Palaeontological Research Group.












