Introduction
Cephalopods, as indicated by Landman et al. (Reference Landman, Davis and Mapes2007), are an important component of marine ecosystems worldwide. They are a well-defined class of Mollusca, and a diverse and highly complex group (Jereb & Roper, Reference Jereb and Roper2005). The cephalopod fauna in the North-west African region includes species that are widely distributed and of high commercial value as fisheries resources (Grant et al., Reference Grant, Griffin and Warren1981; Rocha & Cheikh, Reference Rocha, Cheikh, Valdés and Déniz-González2015; Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017).
The Canary Current Large Marine Ecosystem (CCLME) is one of the four major marine upwelling systems worldwide and the third concerning primary productivity (Valdés & Déniz-González, Reference Valdés and Déniz-González2015). In addition, the CCLME supports the largest fisheries of the African coast. It has an annual fisheries production of ~2–3 million tons (Valdés & Déniz-González, Reference Valdés and Déniz-González2015), including squids, cuttlefishes and octopuses. Thus, one of the most important cephalopod fisheries in the Atlantic Ocean has been developed in its waters, with catches that reach 80,000–120,000 tons per year. Octopuses exported globally under the name Octopus vulgaris Cuvier, 1797, currently considered part of the O. vulgaris species complex (Amor et al., Reference Amor, Norman, Roura, Lite, Gleadall, Reid, Perales-Raya, Lu, Silvey, Vidal, Hochberg, Zheng and Strugnell2017), have been experiencing a high exploitation level since the end of the 1960s in the Cape Blanc area (Inejih et al., Reference Inejih, Ejiwane and Soueilim1995). Today, the individuals found in this area are assigned to O. vulgaris sensu stricto (s. s.), that occurs in the Mediterranean and along the west coast of Africa in the FAO (Food and Agriculture Organization of the United Nations) area 34 (Amor et al., Reference Amor, Norman, Roura, Lite, Gleadall, Reid, Perales-Raya, Lu, Silvey, Vidal, Hochberg, Zheng and Strugnell2017; Sauer et al., Reference Sauer, Gleadall, Downey-Breedt, Doubleday, Gillespie, Haimovici, Ibáñez, Katugin, Leporati, Lipinski, Markaida, Ramos, Rosa, Villanueva, Arguelles, Briceño, Carrasco, Che, Chen, Cisneros, Conners, Crespi-Abril, Kulik, Drobyazin, Emery, Fernández-Álvarez, Furuya, González, Gough, Krishnan, Kumar, Leite, Lu, Mohamed, Nabhitabhata, Noro, Petchkamnerd, Putra, Rocliffe, Sajikumar, Sakaguchi, Samuel, Sasikumar, Wada, Zheng, Tian, Pang and Yamrungrueng2019; Avendaño et al., Reference Avendaño, Roura, Cedillo-Robles, González, Rodríguez-Canul, Velázquez-Abunader and Guerra2020). Currently, two species of the O. vulgaris species complex are identified in the CCLME area: O. vulgaris (s. s.) and O. vulgaris type III, distributed in FAO area 47 (south-western Africa; Sauer et al., Reference Sauer, Gleadall, Downey-Breedt, Doubleday, Gillespie, Haimovici, Ibáñez, Katugin, Leporati, Lipinski, Markaida, Ramos, Rosa, Villanueva, Arguelles, Briceño, Carrasco, Che, Chen, Cisneros, Conners, Crespi-Abril, Kulik, Drobyazin, Emery, Fernández-Álvarez, Furuya, González, Gough, Krishnan, Kumar, Leite, Lu, Mohamed, Nabhitabhata, Noro, Petchkamnerd, Putra, Rocliffe, Sajikumar, Sakaguchi, Samuel, Sasikumar, Wada, Zheng, Tian, Pang and Yamrungrueng2019; Avendaño et al., Reference Avendaño, Roura, Cedillo-Robles, González, Rodríguez-Canul, Velázquez-Abunader and Guerra2020). It is difficult to assess the percentage of octopus in catches off Mauritania, Senegal and The Gambia due to massive under-reporting, lack of records and illegal fishing activities in this region (Belhabib et al., Reference Belhabib, Gascuel, Kane, Harper, Zeller, Pauly, Belhabib, Zeller, Harper and Pauly2012). However, it is still the most exploited group of species in Mauritanian waters, both by artisanal and industrial fisheries (Faure et al., Reference Faure, Inejih, Demarcq and Cury2000; Jouffre et al., Reference Jouffre, Inejih and Caverivière2002; Sauer et al., Reference Sauer, Gleadall, Downey-Breedt, Doubleday, Gillespie, Haimovici, Ibáñez, Katugin, Leporati, Lipinski, Markaida, Ramos, Rosa, Villanueva, Arguelles, Briceño, Carrasco, Che, Chen, Cisneros, Conners, Crespi-Abril, Kulik, Drobyazin, Emery, Fernández-Álvarez, Furuya, González, Gough, Krishnan, Kumar, Leite, Lu, Mohamed, Nabhitabhata, Noro, Petchkamnerd, Putra, Rocliffe, Sajikumar, Sakaguchi, Samuel, Sasikumar, Wada, Zheng, Tian, Pang and Yamrungrueng2019).
Although most of the cephalopod species with commercial value in the region have been well-studied, many aspects of the systematics, biogeography and ecology of other cephalopod species in the CCLME area are practically unknown (Roeleveld, Reference Roeleveld1998; Hoving et al., Reference Hoving, Pérez, Bolstad, Braid, Evans, Fuchs, Judkins, Kelly, Marian, Nakajima, Piatkowski, Reid, Vecchione and Xavier2014; Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017).
The geographic distribution of many cephalopod species in this area is unclear, uncertain or unknown because of the lack of both biodiversity and resource intensive studies. Some surveys of the West African coast were conducted, but the overall records from the Guinea Gulf (Bayer et al., Reference Bayer, Voss and Robins1966; Villanueva et al., Reference Villanueva, Collins, Sánchez and Voss2002) and South Africa (Chun, Reference Chun, Roper and Roper1910; Thiele, Reference Thiele1920) are scattered and incomplete.
During the 19th and 20th centuries, several expeditions with interest in teuthological research were conducted along the North-west African coast: the Talisman (Fischer & Fischer, Reference Fischer and Fischer1892) and the Travailleur (Fischer & Joubin, Reference Fischer and Joubin1906) surveys were conducted from the North-west African coast to Senegal. Thanks to the two expeditions, 18 species (from six families) were added to the 49 species already known by then on the North-eastern Atlantic Ocean coasts (Fischer & Joubin, Reference Fischer and Joubin1906). The Valdivia survey (Chun, Reference Chun, Roper and Roper1910) was a deep-water exploration of the North-east Atlantic and the Indian Ocean, and a total of 52 cephalopod species from 19 families were reported. The Princesse Alice and Hirondelle II surveys (Joubin, Reference Joubin1900, Reference Joubin1920; Joubin & Grimaldi, Reference Joubin and Grimaldi1924) found 55 cephalopod species in 16 families from the Mediterranean Sea to Azores Island, including Morocco and Canary Islands; the Michael Sars survey (Murray & Hjort, Reference Murray and Hjort1912) explored the North Atlantic and found 45 cephalopod species from 18 different families. In 2017, a review of marine biodiversity in the Eastern Central Atlantic (Polidoro et al., Reference Polidoro, Ralph, Michael Harvey, Carpenter, Arnold, Collette, Comeros‐Raynal, De Bruyne, Gon, Harold, Harwell, Hulley, Iwamoto, Knudsen, Lewembe, Linardich, Lindeman, Monteiro, Munroe, Nunoo, Pollock, Poss, Russell, Sayer, Sidibe, Smith‐Vaniz, Stump, Sylla, De Morais, Vié and Williams2017) reported a total of 114 cephalopod species from 33 families in the area.
Many studies have been conducted in Moroccan and Western Sahara waters because of the importance of their fisheries. The Consejo Superior de Investigaciones Científicas (CSIC, Spain) (Allué et al., Reference Allué, Lloris, Rucabado, Guerra and Morales1977) and FAO species identification sheets (Fischer et al., Reference Fischer, Bianchi and Scott1981; Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014), which cover the CECAF 34 area (from the Strait of Gibraltar to Angola) are good examples. The Atlantic waters of Morocco are important for cephalopod trawl fisheries (Suda et al., Reference Suda, Regragui, Sadakane and Machii1996; ARVI, 2013). Especially, the common octopus (O. vulgaris) has been the subject of an important industrial fishery that has used several freezer fleets since the 1960s to capture cuttlefish (Sepia officinalis Linnaeus, 1758) and squid (Loligo vulgaris Lamarck, 1798), as well as octopus (ARVI, 2013).
The coast of Mauritania supports important cephalopod fisheries (Inejih et al., Reference Inejih, Ejiwane and Soueilim1995; Faure et al., Reference Faure, Inejih, Demarcq and Cury2000; Jouffre et al., Reference Jouffre, Inejih and Caverivière2002; Belhabib et al., Reference Belhabib, Gascuel, Kane, Harper, Zeller, Pauly, Belhabib, Zeller, Harper and Pauly2012; Sauer et al., Reference Sauer, Gleadall, Downey-Breedt, Doubleday, Gillespie, Haimovici, Ibáñez, Katugin, Leporati, Lipinski, Markaida, Ramos, Rosa, Villanueva, Arguelles, Briceño, Carrasco, Che, Chen, Cisneros, Conners, Crespi-Abril, Kulik, Drobyazin, Emery, Fernández-Álvarez, Furuya, González, Gough, Krishnan, Kumar, Leite, Lu, Mohamed, Nabhitabhata, Noro, Petchkamnerd, Putra, Rocliffe, Sajikumar, Sakaguchi, Samuel, Sasikumar, Wada, Zheng, Tian, Pang and Yamrungrueng2019). Based on previous publications on cephalopods in the Atlantic (Roper et al., Reference Roper, Sweeney and Nauen1984; Nesis, Reference Nesis1987; Mangold, Reference Mangold1998; Jereb & Roper, Reference Jereb and Roper2005, Reference Jereb and Roper2010; Jereb et al., Reference Jereb, Roper, Norman and Finn2013; Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) and research surveys in the area (Hernández-González et al., Reference Hernández-González, Faraj, Balguerías, Belcaid, Burgos, Cansado, Fernández, González, Jiménez, Manchih, Meiners, Muñoz, Nuño, Presas, Ramos, Salmerón, Settih and Soto2006, Reference Hernández-González, Bouzouma, Burgos, Hernández and Cheikna2008; Hernández-González, Reference Hernández-González2007; Ramos et al., Reference Ramos, Sanz and Ramil2017; Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017), a total of 132 cephalopod species belonging to 39 families have been reported from Mauritanian waters.
There is no available information on cephalopod diversity and biology in Senegalese and Gambian waters (like several other countries in the area), except for the most important commercial species (Gulland & García, Reference Gulland, García and May1984; Caverivière et al., Reference Caverivière, Domain and Diallo1999; Darboe & Mendy, Reference Darboe and Mendy2002; Diallo et al., Reference Diallo, Jouffre, Caverivière and Thiam2002; Tandstad & Caramelo, Reference Tandstad and Caramelo2011; FAO, 2019). There is limited information on cuttlefishes (mainly S. officinalis), bobtail squids (Sepiolidae Leach, 1817), octopuses (mainly O. vulgaris) and squids (Loliginidae Lesueur, 1821, mainly L. vulgaris, and Ommastrephidae Steenstrup, 1857 species) (Tandstad & Caramelo, Reference Tandstad and Caramelo2011). These countries have difficulties in regulating the effort or number of fishing captures, and it is probable that most of these species are overexploited (FAO, 2019). Because of this lack of information in Senegal and The Gambia, it is clear that more prospective surveys and studies are needed to not only understand cephalopod diversity but also other potential commercial species in the region.
The cephalopod diversity in Guinean waters is poorly understood. Two surveys by the R/V ‘Dr Fridtjof Nansen’ at the beginning of the 21st century found only four cephalopod species: the cuttlefish Sepia hierredda Rang, 1835; squids Illex coindetii (Vérany, 1839) and Todaropsis eblanae (Ball, 1841) and the Lilliput longarm octopus, Macrotritopus defilippi (Verany, 1851). Both cruise reports established that cephalopods contributed only marginally to the total catch from this region (Huse et al., Reference Huse, Alvheim and Turay2006; Mehl et al., Reference Mehl, Lundsør, Turay, Sei and Lamptey2007).
Between 2004 and 2012, the Spanish Institute of Oceanography (IEO) and the Institute of Marine Research (IMR) of Norway conducted 12 multidisciplinary programmes along the North-west African margin in the waters off Morocco, Western Sahara, Mauritania, Senegal, The Gambia, Guinea–Bissau, Guinea, and Cabo Verde. In this intensive sampling programme for benthos, quantitative data, environmental parameters of the water column and seabed, an extended collection of wildlife specimens (both pelagic and benthic) and databases that were only partially studied were obtained. Their study will allow us to acquire a more complete view on cephalopod biodiversity, composition and distribution in the coast of Africa and information on the ecosystems and natural marine resources of the area.
In the technical reports of these CCLME surveys in 2011 and 2012 (Krakstad et al., Reference Krakstad, Michalsen, Alvheim, Zaera, Bagøien, Krafft, García-Isarch, Ramil and Van Waerebeek2011, Reference Krakstad, Michalsen, Høines, Alvheim, Zaera, Olsen and García–Isarch2012) which included the EEZ (exclusive economic zone) of seven countries – Cape Verde, Guinea, Guinea–Bissau, Senegal, The Gambia, Mauritania and Morocco – the following 12 cephalopod species were reported: Alloteuthis africana Adam, 1950, Octopoteuthis megaptera (Verrill, 1885), Opisthoteuthis agassizii Verrill, 1883, I. coindetii and T. eblanae; cuttlefishes Sepia elegans Blainville, 1827, Sepia hieronis (Robson, Reference Robson1924), S. hierredda, S. officinalis, Sepia orbignyana Férussac, 1826 and Sepiella ornata (Rang, 1837) and the common octopus, O. vulgaris.
Additionally, several taxonomic studies on cephalopods, including species present in the CCLME area, have been performed. These monographic publications include the genus Enoploteuthis d'Orbigny, 1842 (Roper, Reference Roper1966; Young & Harman, Reference Young and Harman1998), families Joubiniteuthidae Naef, 1922 and Cycloteuthidae Naef, 1923 in the North Atlantic (Young & Roper, Reference Young and Roper1969a, Reference Young and Roper1969b) and the subfamily Rossiinae Appellöf, 1898 (Boletzky, Reference Boletzky1971). Cephalopod species of interest to fisheries in the area have been reported in the identification sheets for zone 34 of the Fishery Committee for the Eastern Central Atlantic (CECAF, FAO; Allué et al., Reference Allué, Lloris, Rucabado, Guerra and Morales1977; Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). Finally, other specific publications on cephalopods can be found for the oceanic squids Sthenoteuthis pteropus (Steenstrup, 1855) in the Atlantic Ocean (Zuyev et al., Reference Zuyev, Nigmatullin, Chesalin and Nesis2002), Lepidoteuthis grimaldii Joubin, 1895 in the Canary Islands (Escánez et al., Reference Escánez, Guerra, Rocha and Lozano-Soldevilla2017) and families Histioteuthidae Verrill, 1881, Cranchiidae Prosch, 1847 and Octopodidae d'Orbigny, 1840 in the Azores Islands (Gomes-Pereira et al., Reference Gomes-Pereira, Gonçalves and Clarke2016).
In this review, we focused on the taxonomic study of the cephalopod collections obtained from the water off Morocco, Western Sahara and Guinea–Bissau during the Spanish programmes (2004–2008) as well as those collected during the two regional programmes of FAO, CCLME–2011 and CCLME–2012, in the Strait of Gibraltar and that border Sierra Leone. These collections represent an exceptional source of information that will provide a global view of the biodiversity, composition and distribution of cephalopods from the North-west coast of Africa. In addition to specimen identification, inventory and characterization of the teuthological fauna of the North-west African coast at the regional level were performed. Special emphasis was placed on the study of the less-known species and those whose known distributional ranges have been expanded by this study.
Materials and methods
The surveys
The specimens were obtained during 10 bottom trawl surveys developed between 2004 and 2012 by the IEO of Spain and the IMR of Norway. These surveys (Table 1) were part of the EcoAfrik project (Biodiversity of benthic ecosystems of Africa) by the IEO, within the programme of study of African fishery resources (CECAF, Committee for Eastern Central Atlantic Fisheries). Waters of the shelf and continental slope were explored by the Spanish R/V ‘Vizconde de Eza’ and Norwegian ‘Dr Fridtjof Nansen’ vessels, from the Strait of Gibraltar in Moroccan waters (~36°N) to the northern border of Sierra Leone waters (9°N), and the EEZ of seven countries (Morocco, Western Sahara, Mauritania, Senegal, The Gambia, Guinea–Bissau and Guinea), to evaluate the pelagic and demersal resources and reference the state of the ecosystem at the regional level (Krakstad et al., Reference Krakstad, Michalsen, Alvheim, Zaera, Bagøien, Krafft, García-Isarch, Ramil and Van Waerebeek2011, Reference Krakstad, Michalsen, Høines, Alvheim, Zaera, Olsen and García–Isarch2012) between 20 and 2000 m. Maroc surveys were conducted in Morocco and Western Sahara waters (Ramos et al., Reference Ramos, Faraj, Balguerías, Belcaid, Burgos, Gómez, González, Hakim, Hernández, Manchih, Meiners, Ramil, Salmerón, Sanz and Settih2005; Hernández-González et al., Reference Hernández-González, Faraj, Balguerías, Belcaid, Burgos, Cansado, Fernández, González, Jiménez, Manchih, Meiners, Muñoz, Nuño, Presas, Ramos, Salmerón, Settih and Soto2006), Maurit surveys in Mauritanian waters (Hernández-González et al., Reference Hernández-González, Faraj, Balguerías, Belcaid, Burgos, Cansado, Fernández, González, Jiménez, Manchih, Meiners, Muñoz, Nuño, Presas, Ramos, Salmerón, Settih and Soto2006, Reference Hernández-González, Bouzouma, Burgos, Hernández and Cheikna2008; Hernández-González, Reference Hernández-González2007; Ramos et al., Reference Ramos, Sanz and Ramil2017), Bissau–0810 survey was conducted in Guinea–Bissau waters (García-Isarch et al., Reference García-Isarch, Burgos, Sobrino, Almeida, Barry, Nahada, Funny and Gómez2009), and the two FAO regional surveys (CCLME surveys) were conducted across the CCLME region between the Strait of Gibraltar and the waters of the border between Guinea and Sierra Leone. Information about the surveys is available in Table 1.
Table 1. Summary of the characteristics and main objectives of the studied surveys

The trawls used in the surveys were a commercial trawl (Lofoten type) for Maroc and Maurit surveys (Hernández-González et al., Reference Hernández-González, Faraj, Balguerías, Belcaid, Burgos, Cansado, Fernández, González, Jiménez, Manchih, Meiners, Muñoz, Nuño, Presas, Ramos, Salmerón, Settih and Soto2006), a commercial Conakry cod-end type trawl for Bissau–0810 (García-Isarch et al., Reference García-Isarch, Burgos, Sobrino, Almeida, Barry, Nahada, Funny and Gómez2009), and a Gisund super bottom trawl for the regional CCLME surveys (Krakstad et al., Reference Krakstad, Michalsen, Alvheim, Zaera, Bagøien, Krafft, García-Isarch, Ramil and Van Waerebeek2011, Reference Krakstad, Michalsen, Høines, Alvheim, Zaera, Olsen and García–Isarch2012).
As a result, quantitative data from 1298 sampling stations (Figure 1) were obtained and an important collection of cephalopods was preserved, which have been deposited in the Reference Collections of the IEO of the Canary Islands and Málaga, and the Cephalopod Laboratory of the University of Vigo (Faculty of Marine Sciences). With regard to the four Maurit surveys, this study is complementary to the previous one performed by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017).
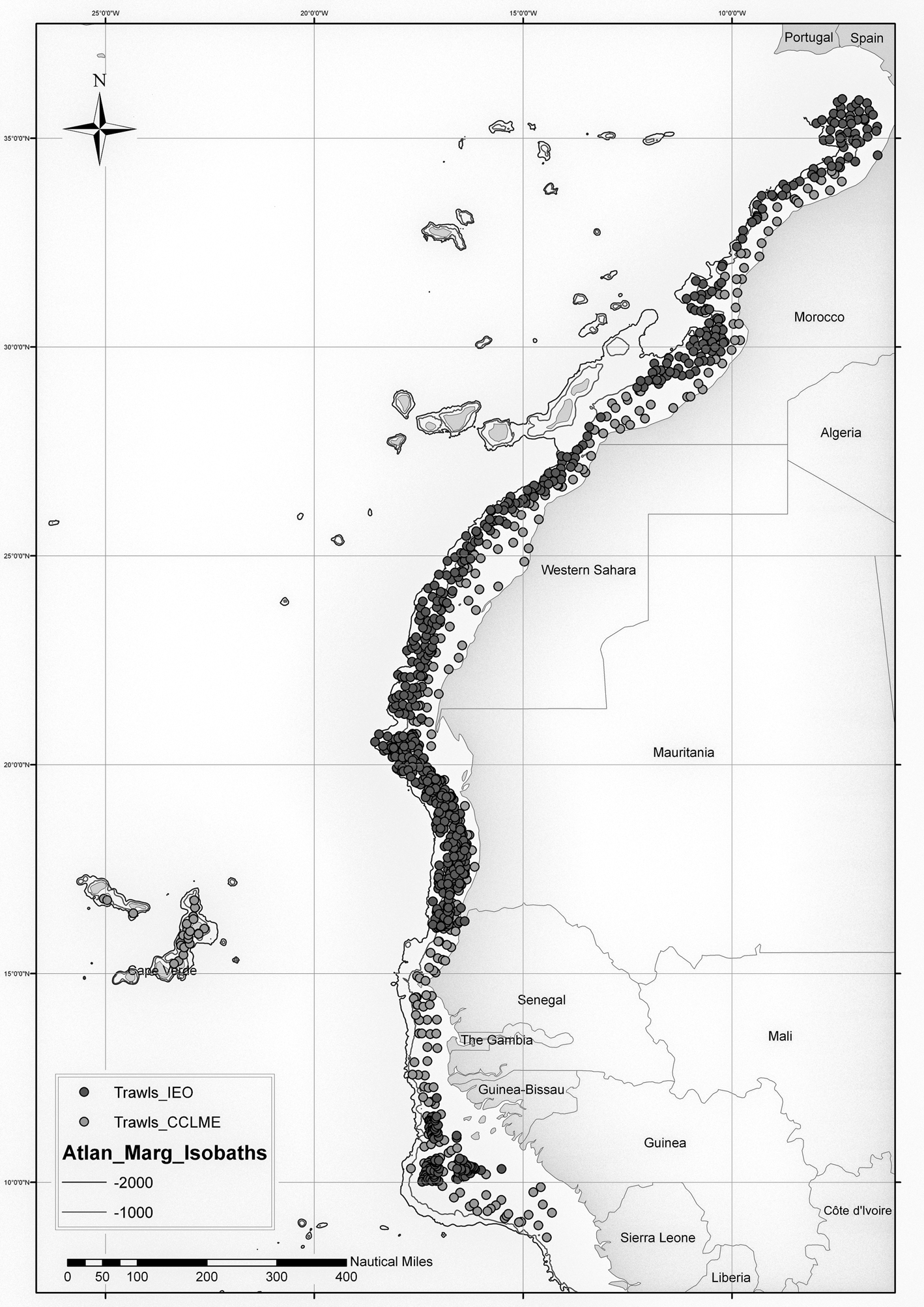
Fig. 1. Map of CCLME campaign stations. In dark grey, the stations carried out by the IEO campaigns (Maroc, Maurit and Bissau); in light grey, the regional campaigns of the CCLME (CCLME). The isobaths of −1000 m and −2000 m of the Atlantic margin are shown.
Taxonomic analysis
The study was performed at the University of Vigo (Vigo, Spain) and the Spanish Institute of Oceanography of the Canary Islands (Tenerife, Spain). The specimens identified in the present paper were those preserved for later study during the surveys because it was not possible to identify them soon after capture (except for the specimens from Bissau–0810 which were previously identified on board and already thoroughly checked in the laboratory). Identification included morphological analyses of each specimen. The specimens were preserved during the campaigns in 4% formalin in seawater. Upon examination, the preservative was replaced by successive washings in fresh water and ethyl alcohol at different increasing concentrations (25–40–70%), until its final conservation in 70–80% alcohol in fresh water. The deteriorated specimens were kept in 4–7% formalin. The individuals were identified using their external taxonomic characters and morphometry. For taxonomic identification to the genus and species levels, cephalopod descriptions and taxonomic keys published by Robson (Reference Robson1929, Reference Robson1932), Nesis (Reference Nesis1987), Guerra (Reference Guerra and Ramos1992), Okutani & Clarke (Reference Okutani, Clarke, Sweeney, Roper, Mangold, Clarke and Boletzky1992), Bello (Reference Bello1995, Reference Bello2013, Reference Bello2015), Muus (Reference Muus2002), Gleadall (Reference Gleadall2004), Jereb & Roper (Reference Jereb and Roper2005, Reference Jereb and Roper2010), Allcock et al. (Reference Allcock, Strugnell, Ruggiero and Collins2006), Bolstad (Reference Bolstad2010), Gleadall et al. (Reference Gleadall, Guerrero–Kommritz, Hochberg and Laptikhovsky2010), Jereb et al. (Reference Jereb, Roper, Norman and Finn2013), Guerra et al. (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) and Bolstad et al. (Reference Bolstad, Braid, Strugnell, Lindgren, Lischka, Kubodera, Laptikhovsky and Roura2018) were used. The specimens in poor condition were identified by their mandibles or internal shells using specific literature (Voss, Reference Voss1956; Roper, Reference Roper1966; Lipinski, Reference Lipinski1983; Clarke, Reference Clarke1986; Pérez-Gándaras, Reference Pérez-Gándaras1986; Guerra et al., Reference Guerra, Pérez-Losada, Rocha and Sanjuán2001; Lu & Ickeringill, Reference Lu and Ickeringill2002; Xavier & Cherel, Reference Xavier and Cherel2009; Bolstad, Reference Bolstad2010). We also consulted websites that specialize in taxonomy, for example, World Register of Marine Species (WoRMS), Tree of Life (ToL), Encyclopedia of Life (EOL) and Global Biodiversity Information Facility (GBIF). The classification by Young et al. (Reference Young, Vecchione and Mangold2019), available on the ToL website, was followed for the taxonomic classification of the specimens. The recommendations of Roper & Voss (Reference Roper and Voss1983) were taken into account for the measurement, indexing and characterization of the specimens. The lesser-known species and those whose range of distribution has been extended were also described using the guidelines provided by Roper & Voss (Reference Roper and Voss1983). Photographs of the fresh specimens taken during the surveys were used to determine their colouration patterns and general appearance. Then, each species was re-tagged, and their peculiar identification characters were photographed. Finally, after an exhaustive review of the existing literature on cephalopods, an updated checklist for the species recorded in the study area was generated.
Results
The cephalopods in the CCLME region
After an exhaustive review of the existing cephalopod literature and new data obtained from the surveys, an updated checklist of 138 species for the CCLME area was generated (Table 2). We have documented the geographic ranges of a total of 23 species, and seven others were removed from the initial list because their presence is uncertain in the area or changes in the information on their taxonomy or distribution have occurred.
Table 2. Checklist of all specimens recorded and found in the CCLME area, arranged alphabetically by order and family

All, CCLME area; Austr, Australia; Az, Azores Islands; Bi, Guinea–Bissau; Can, Canary Islands; CAtl, Central Atlantic; Cosm Temp, Cosmopolitan temperate; CV, Cabo Verde Islands; EAtl, Eastern Atlantic; Ga, Gambia; Gui, Guinea; GuiG, Guinea Gulf; Jap, Japan; Ma, Mauritania; Mad, Madeira Islands; Med, Mediterranean; Mo, Morocco; Nam, Namibia; NW Atl, North-western Atlantic; Port, Portugal; Sa, Western Sahara; SAf, South Africa; Se, Senegal.
In the CCLME area, a total of 378,377 cephalopod specimens were collected from 1247 trawl stations (Table 1). From the 300 specimens preserved for posterior identification, we found 65 different species in 23 families. Table 3 includes the specimen information by survey (zone, numerosity of taxa examined, species and families), and Table 4 the number of species and families by cephalopod order. The presence/absence of each species is listed by countries in the area (Table 2).
Table 3. Number of examined specimens (N), species and families identified by survey and zone

Table 4. Cephalopod specimens sampled from 1247 commercial trawls in the CCLME area

Of 193 specimens collected from Moroccan waters, 52 species belonging to 23 families were identified: two species in the order Myopsida Naef, 1916 (3.8% of the total species from Moroccan waters), 21 from Oegopsida Orbigny, 1845 (40.4%), seven species from Sepiolida Keferstein, 1866 (13.3%), three from Sepiidae Keferstein, 1866 (5.7%), 17 species (32.7%) from Octopoda Leach, 1818 and one species from Spirulida Haeckel, 1896 (1.9%). The families with the largest number of species found in Moroccan waters were Octopodidae, with five species (9.6% of the total), followed by Histioteuthidae, with four species (7.7%).
Guerra et al. (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) reported 88 cephalopod species in Moroccan waters that were classified into 30 families and six orders. In the checklist for Moroccan waters, three newly reported cephalopod families (Mastigoteuthidae, Lepidoteuthidae and Enteroctopodidae) and 15 new species have been included: one in the order Sepiolida (Austrorossia mastigophora (Chun, 1915)), seven in the order Oegopsida (Abralia (Heterabralia) siedleckyi; Abralia (Asteroteuthis) veranyi; Histioteuthis meleagroteuthis (Chun, Reference Chun, Roper and Roper1910); Stigmatoteuthis arcturi; Magnoteuthis magna; Octopoteuthis megaptera and L. grimaldii) and seven in the order Octopoda (C. murrayi; Eledone cirrhosa (Lamarck, 1798); M. fuscus; M. januarii; O. salutii; Scaeurgus unicirrhus (Delle Chiaje, 1830) and Opisthoteuthis agassizii?).
In waters off Senegal, 13 species belonging to 10 families were caught during the cruises (Tables 2 and 3): one in the orders Sepiida and Myopsida (7.7%), seven in the order Oegopsida (53.8%) and four in the order Octopoda (30.8%). Thus, families with the greatest number of species found in Senegalese waters were Histioteuthidae, Cranchiidae and Enteroctopodidae Strugnell, Norman, Vecchione, Guzik & Allcock 2013, with two species each and 15.4% of the total Senegalese species. Taking O. vulgaris into account, a total of 14 cephalopod species from 10 families are the total number of cephalopods known in Senegalese waters.
The Gambia has no specific cephalopod literature. The only available information was derived from fisheries data on octopuses NEI (Not Elsewhere Included: Octopodidae, with maximum attention to O. vulgaris), cuttlefish (Sepia spp.; overall, S. officinalis), bobtail squids NEI (Sepiolidae) and various squids NEI (Loliginidae; overall, L. vulgaris and Ommastrephidae) (Darboe & Mendy, Reference Darboe and Mendy2002; Tandstad & Caramelo, Reference Tandstad and Caramelo2011; FAO, 2019). In the Gambia waters, only one species was collected: Vitreledonella richardi Joubin, 1918, belonging to the family Amphitretidae Hoyle, 1885 (Table 2), with two specimens. With the addition of V. richardi to the Gambia fauna, a total of four cephalopod species have been identified in these waters (O. vulgaris, S. officinalis, L. vulgaris and V. richardi) (Table 3).
In Guinea–Bissau waters, to date, a total of 13 cephalopod species in five families had been reported (Pereira, Reference Pereira1993; Huse et al., Reference Huse, Alvheim and Turay2006; Mehl et al., Reference Mehl, Lundsør, Turay, Sei and Lamptey2007; Barri, Reference Barri2008; Heileman, Reference Heileman, Sherman and Hempel2009; Fernández-Caballero, Reference Fernández-Caballero2014; FAO, 2019). Guerra et al. (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) reported six cephalopod species specifically in Guinea–Bissau waters that were classified into five families and two orders. In addition, in the same work, another 74 species, belonging to 24 families in five orders, seems to be close in distribution to Guinea–Bissau waters, and they could be present here. In the present work, specimens of 25 species belonging to 14 families have been analysed. Four species (16.0%) correspond to the order Sepiida, two to Sepiolida (8.0%), three to Myopsida (12.0%), seven to Oegopsida (28.0%) and nine to Octopoda (36.0%). The most speciose family in waters off Guinea–Bissau was Sepiidae, with four species (16.0% of the total), followed by Loliginidae and Ommastrephidae, with three species each (12.0%). In consequence, the number of cephalopod species in waters off Guinea–Bissau increased to 20, with the addition of Eledone moschata (Lamarck, 1798), M. defilippi and O. vulgaris from the order Octopoda; L. vulgaris from Myopsida; Todarodes sagittatus (Lamarck, 1798) from Oegopsida and S. officinalis from Sepiida.
Very little diversity data have been obtained from Guinean waters. Only some survey data are available for this country (Huse et al., Reference Huse, Alvheim and Turay2006; Mehl et al., Reference Mehl, Lundsør, Turay, Sei and Lamptey2007), and four cephalopod species had been reported: the cuttlefishes S. hieronis and S. hierredda, squids I. coindetii and T. eblanae and octopus M. defilippi (Mehl et al., Reference Mehl, Lundsør, Turay, Sei and Lamptey2007; Krakstad et al., Reference Krakstad, Michalsen, Alvheim, Zaera, Bagøien, Krafft, García-Isarch, Ramil and Van Waerebeek2011, Reference Krakstad, Michalsen, Høines, Alvheim, Zaera, Olsen and García–Isarch2012). Some other cephalopod groups have been reported as the prey of elasmobranchs in Guinean waters: Theuthidae indet., Sepia sp., Octopus sp. and Rossia sp. Owen, 1835 (Patokina & Litvinov, Reference Patokina and Litvinov2005). In the Guinean waters only two species, S. ornata and S. hierredda, belonging to the family Sepiidae, have been collected (Table 2). Thus, the total number of cephalopod species off Guinea is five, and they belong to three families.
Species of special concern
Order Sepiolida
In the family Sepiolidae Leach, 1817, two specimens of A. mastigophora were identified for the first time in Western Sahara waters (Appendix 1): a female (3.5 cm mantle length (ML)) and a male (2.5 cm ML). The specimens were identified based on diagnostic characters described in Guerra et al. (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014): the mantle margin slightly retracted (especially on the dorsal side), without projecting corners, a tentacle club dorsally curved like a horn with very small suckers (30–40 in each row) and pairs of enlarged biserial suckers on ventral, ventrolateral and dorsolateral arms in males (8, 8 and 6 pairs, respectively).
In the subfamily Sepiolinae Appellöf, 1898, a total of 13 specimens of Sepiola atlantica d'Orbigny, 1845 were collected (Appendix 1), nine of them caught in Western Sahara waters. The specimens were identified based on the following diagnostic characters: the anteroventral edge of mantle undulate, without deep incision, and a pair of kidney-shaped light organs present inside the mantle cavity on each side of ink sac. In the male, the hectocotylus (left dorsal arm) is strongly bent starting at the middle. The distal part of the hectocotylus has two groups of markedly enlarged suckers in dorsal row, in proximal and midway position. The midway position group presents 4–5 greatly enlarged suckers with fused pedicels and, in the base of the arm, a large swollen bulb, with the copulatory organ in the form of secondary basal lobes. According to Bello (Reference Bello2013), the specimens had eight rows of suckers in the tentacular club, which distinguishes the species from S. tridens de Heij & Goud, 2010 and confirms it as S. atlantica. Also, this species is characterized by having two sucker series on arms IV, which abruptly change into minute suckers arranged in 4–6 transverse series on tips, which are long and finger-like (Nesis, Reference Nesis1987; Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014).
Superfamily Bathyteuthoidea Vecchione, Young and Sweeney, 2004
A female Chtenopteryx sicula (Verany, 1851) (Chtenopterygidae Grimpe, 1922) was identified in Western Sahara waters, with 4.6 cm ML (Appendix 1). The specimen was identified according to an indisputable diagnostic character: large photophores on the ventral surface of eyeballs, a feature present in C. sicula and absent in other congeneric species as C. canariensis specimens (Salcedo-Vargas & Guerrero-Kommritz, Reference Salcedo-Vargas and Guerrero-Kommritz2000; Escánez et al., Reference Escánez, González and Guerra2012, Reference Escánez, Roura, Riera, González and Guerra2018; Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). The female presents a mantle width (MW) of about 2 cm (42.5% ML) as well as the characteristic number of 4 series of suckers in arms I-II-III and a tentacular club with 8–12 series of suckers. Also, the characteristic tentacular club curvature present in the species was visible.
In the family Bathyteuthidae Pfeffer 1900, Bathyteuthis abyssicola Hoyle, 1885 was found in Moroccan waters. This specimen was in very bad condition, but the eyeballs, gladius and mandibles were recovered (Figure 2). The specimen was identified based on figures and descriptions of the gladius (Roper, Reference Roper1969; Toll, Reference Toll1998) and mandibles (Clarke, Reference Clarke1986; Lu & Ickeringill, Reference Lu and Ickeringill2002; Xavier & Cherel, Reference Xavier and Cherel2009) by Guerra (personal communication). However, the morphometric relationships between the beak measurements did not match exactly those described by Clarke (Reference Clarke1986). The wing length (a) was 25–26 mm, length of the rostral edge visible in profile (b) was 89–93 mm and edge-wing ratio (b/a) was 3.4, which is larger than 2–3 as reported by Clarke (Reference Clarke1986). The hood length in the midline (g) was 68 mm, and the hood to edge ratio (g/a) was 2.7, which is larger than it should be (2–2.5) according to the available literature; the distance in profile from the rostral tip to the interaction of the rostral edge with the wing fold (h) was 40 mm, and the rostral base ratio (h/a) was 1.3, which is less than it should be (>2). It could be a range extension of morphological variability of the beak of this species or population differences.

Fig. 2. Bathyteuthis abyssicola Hoyle, 1885. Lower beak (A), upper beak (B) and gladius (C). Maroc–0511 survey. Scale: each line, 1 cm. (A and B, © IEO; C, © Amanda Luna).
Order Oegopsida
In the family Cranchiidae, five specimens of Taonius pavo (Lesueur, 1821) were found in Senegalese waters (Appendix 1). The specimens were identified as T. pavo based on the following features (Nesis, Reference Nesis1987; Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014): mantle very long, slender, narrow and cone-shaped; mantle fused with head in the nuchal region; small head and bulbous eyes; fins lanceolate, extending half the ML; arms with biserial, spherical suckers, and tentacles a little longer than arms; a tentacular club with four series of suckers (the specimen was an adult), and the manus sucker rings with two large, central, hook-like teeth. As indicated by Young (Reference Young2014) a secondary ring tooth of manus suckers was absent in the specimens. According to Young (Reference Young2014), our specimens had the large club suckers of the marginal series of manus not laterally compressed, with long, pointed teeth on distal and lateral margins of the sucker ring, and a distinct carpal cluster at the base of manus with not very obvious six smooth-ringed and matching knobs.
In the family Enoploteuthidae Pfeffer, 1900, a specimen of Abralia (Pygmabralia) redfieldi Voss, 1955 female (3.5 cm ML) was identified in Western Sahara waters from a depth of 74 m (Appendix 1). This species was identified based on the following diagnostic characters: pink buccal membrane characteristic of the genus Abralia; 5 rounded ventral optic light organs (the first, third and fifth larger than the second and fourth); and 3–4 hooks on the tentacular club. The specimens have diagnostic characters such as the absence of large black globular photophores on tips of ventral arms and ventral surface of the mantle and head covered with numerous scattered light organs but leaving a bare stripe along the ventral mantle midline (Nesis, Reference Nesis1987; Golub, Reference Golub2001; Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014).
A total of 13 A. (H.) siedleckyi Lipinski, Reference Lipinski1983 specimens were found onboard the Maroc–0611 survey (Appendix 1), with an ML range of 3.4–4.1 cm. The distance between the dactylus and manus is similar to each other in this species, and, according to Nesis (Reference Nesis1987), the two rows of tiny suckers on the tips of arms I, II and III clearly distinguish it from its relative A. (A.) veranyi Rüppell, 1844, characters that we could clearly observe. Other important morphological features that our specimens had were the three hooks on the tentacular club and photophore pattern on the ventral region of the eyeball, and five complex photophores, consistent with the description by Sajikumar et al. (Reference Sajikumar, Lipinski, Venkatesan, Sasikumar and Mohamed2018): two terminal oval, creamy white, opaque organs (posterior is extra-large) and three intermediate orange organs. The dorsal part of the eyelid bore 16 black photophores. To date, the maximum ML for this mesopelagic species was 3.8 cm (Hidaka & Kubodera, Reference Hidaka and Kubodera2000); thus, the ML range has increased for the species.
Several specimens of A. (A.) veranyi were found in Moroccan and Western Sahara waters as well as the Guinea–Bissau, Senegal and Mauritania coasts, with an ML range of 2.5–5 cm. The distinctive features of this species were the ventral surface of mantle, head and arms covered with numerous scattered light organs; minute distal suckers in three or four series in the arms and five optic light organs with the terminal two oval and larger than the middle three rounded ones (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). This species is distinctive because of other features found in the studied specimens, namely, conical mantle with sagittate posterior fins; arms I to III with biserial suckers proximally, some of them changed into hooks; left ventral arm hectocotylized, with a pair of fleshy distal flaps and tentacular club with three or four (three were found) hooks and a dorsal membrane (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014).
In the family Histioteuthidae, a juvenile of S. arcturi Robson, 1948, specimen of 2.4 cm ML was obtained in North Moroccan waters at a depth of 995 m (Appendix 1). The specimen, which was in poor condition as it does not have tentacles or web, was identified by the papillated skin (Voss et al., Reference Voss, Nesis and Rodhouse1998); dorsal pad of funnel organ sculptured with a median ridge down each arm, and the distal portion of the median ridge on arms of dorsal pad funnel organ expanded into a distinct flap (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). According to Voss et al.'s (Reference Voss, Nesis and Rodhouse1998) and Guerra et al.'s (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) descriptions, other characters present in the studied juvenile were the lack of distinct terminal light organs on arms and presence of 17 large light organs in a circle around the margin of the right eyelid.
In the family Lepidoteuthidae Pfeffer, 1912, a male of L. grimaldii (19 cm ML) was collected from South Moroccan waters (Appendix 1) at a depth of 843 m. According to Clarke & Maul (Reference Clarke and Maul1962), the specimen was identified by the presence of dermal cushions covering the mantle together with the lack of the tentacles (typical in subadult/adult stage of this species) and the presence of a single hook near the base of each arm II (feature characteristic in males of this species).
In the family Mastigoteuthidae Verrill, 1881, two specimens of M. magna (Joubin, 1913) were found: one in South Moroccan waters (12.3 cm ML) and the other off Western Sahara waters (7.0 cm ML) (Appendix 1). The features (Figure 3) are consistent with the original description by Joubin (Reference Joubin1920): club suckers minute with smooth inner rings; arm suckers increasing in size from base to sucker pair 12 or 13 and next 15 pairs of the same size; then, size diminishes to tip, but the largest suckers of arms IV larger than those of other arms. The funnel-locking apparatus as ‘auricular-like groove’ and mantle component a ‘rectilinear ridge narrow anteriorly and slightly wider posteriorly’ (Joubin, Reference Joubin1920) were observed.
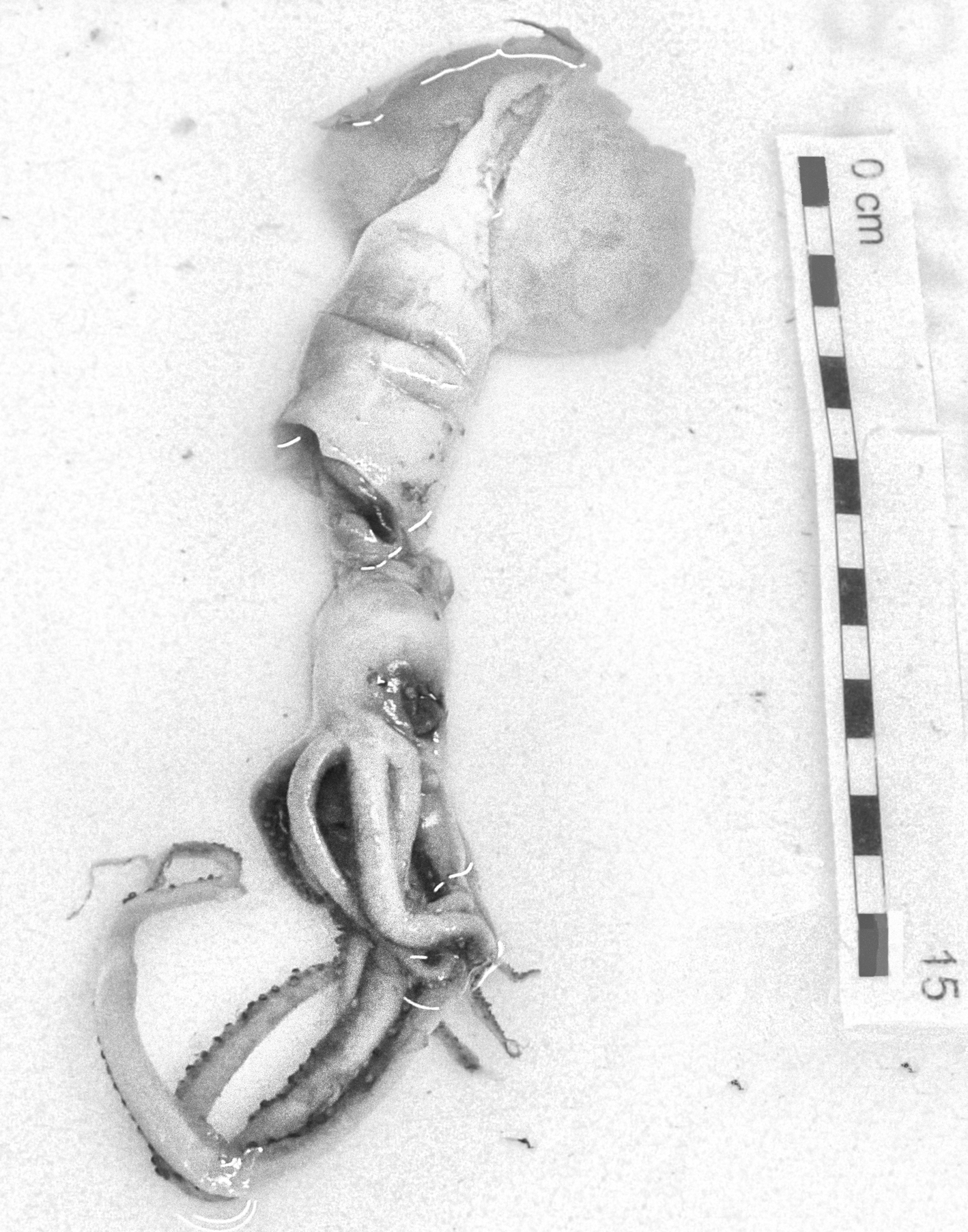
Fig. 3. Magnoteuthis magna (Joubin, 1913). Dorsal view. Maroc–0511 survey. Scale: each line, 1 cm. (© Amanda Luna).
In the family Octopoteuthidae Berry, 1912, six O. megaptera individuals were found: one in South Moroccan waters (19.0 cm ML), two off the Western Sahara coast (4.5 and 6.0 cm ML), one in water off Mauritania (9.0 cm ML), and two in Senegalese coast (8.0 and 12.0 cm ML) (Appendix 1). Three of the records exceed 1100 m depth (1367–1820 m). The most conspicuous diagnostic characters of this octopoteuthid that we could observe in our specimens were the ventral pair of light organs at the posterior end of the mantle, with transparent mantle tissue covering them; fin length about 75% ML, with fin not reaching the posterior end of the mantle; and long, acuminated tail flattened from above, with narrow lateral fringes. Other observed characters were a pair of light organs inside the mantle cavity on both sides of the ink sac and arms short and thick, with several ends broken off and biserial hooks covered by a hood of soft tissue.
In the family Onychoteuthidae Gray, 1849, five specimens of Ancistroteuthis lichtensteinii (Férussac, 1835) were identified in Moroccan waters (8.0 and 9.2 cm ML), Western Sahara (17.0 and 18.8 cm ML) and Guinea–Bissau (female, 12.0 cm ML) coast (Appendix 1). According to known distribution (Bolstad, Reference Bolstad2010) the Guinea–Bissau record expands its known distribution south. The specimens were identified by the sagittate fin width (55–65% ML) and the presence of 10 occipital folds on either side of the head. Other diagnostic features of A. lichtensteinii were: distal suckers on club restricted to a terminal pad, and no gladius or photophores visible beneath the skin in the dorsal mantle midline. The specimens also have a double medial series of hooks (20–22) on the manus, 16–18 small suckers on dactylus and a carpal-fixing apparatus on club elliptical with 9–12 suckers. The small posterior patch of tissue of the oval, opaque area on the ventral covering of the eye was present; it was thought to be photogenic tissue by Kubodera et al. (Reference Kubodera, Piatkowski, Okutani and Clarke1998) and likely to be iridescent (but not photogenic) by Vecchione et al. (Reference Vecchione, Young and Tsuchiya2010b). All the specimens reported in the present study were A. lichtensteinii (s. s.) (Type A by Kubodera et al. (Reference Kubodera, Piatkowski, Okutani and Clarke1998), except the Guinea–Bissau specimen (11.5 cm ML) that seems to be Type B by Kubodera et al. (Reference Kubodera, Piatkowski, Okutani and Clarke1998)) because of the rhomboidal fin, the number of nuchal folds (6), fin length (6.8 cm) and Fin Length Index (59.1% ML).
Another onychoteuthid species identified was Onykia robsoni (Adam, 1962). The specimen was a female with 39.0 cm ML (Figure 4). The record of the specimen expanded the known geographic distribution range to Guinea–Bissau waters (Appendix 1). The observed characters were consistent with the description provided by Bolstad (Reference Bolstad2010): rugose structure of mantle epidermis, with large, soft, round, well separated, blister-like warts, the epidermis of head and arm-bases smooth; photophores and secondary occipital folds absent; long sagittate fins, with length ~60% ML (51.3% ML in the studied specimen), width ~45.0% ML (46.2% ML) and attenuate posteriorly; funnel groove U-shaped, with Y-shaped ridge present; head noticeably narrower than mantle; 26–28 hooks present on adult tentacle club; lateral grooves on the manus hook claws and gladius not visible dorsally through the mantle.
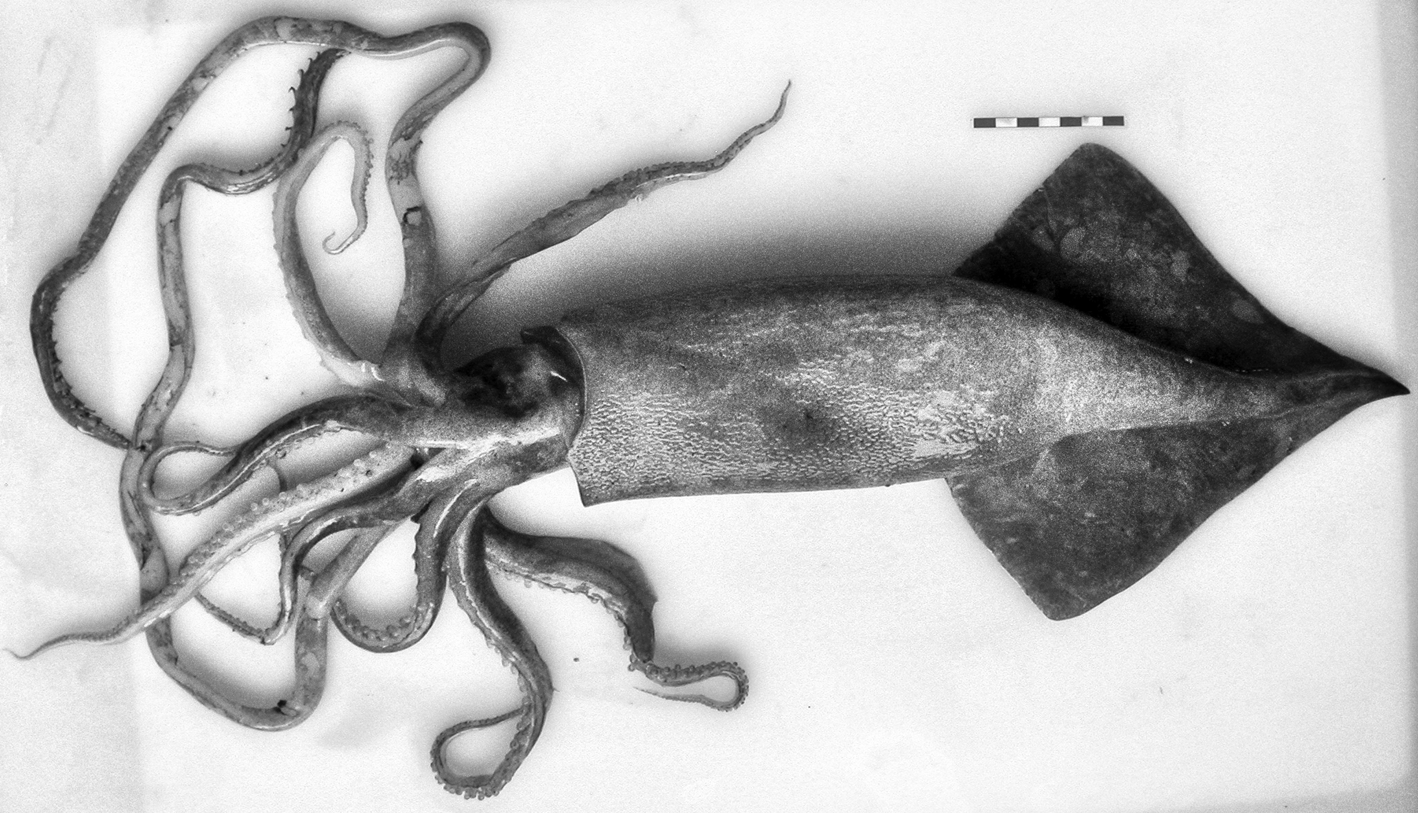
Fig. 4. Onykia robsoni (Adam, 1962). Dorsal view. Bissau–0810 survey. Scale: each line, 1 cm. © IEO.
Order Octopoda
In the order Octopoda, family Cirroteuthidae Keferstein, 1866, one specimen of Cirrothauma murrayi Chun, 1911 was found in South Moroccan waters (Appendix 1). The animal was caught at a depth of 1554 m; it was in very bad condition, which caused difficulties in the measurements. The specimen had a gelatinous body elongate in anterior-posterior axis; its eyes are reduced to simple open cups exposed to the exterior and embedded within gelatinous tissue and look like small dark balls, lacking lens or iris. These poorly developed eyes indicate that it may be nearly blind and unlikely to be able to form a focused image (Jereb et al., Reference Jereb, Roper, Norman and Finn2013). It had a pair of large fins on the mantle, attached to the middle of the body, closer to the head than the posterior mantle end, exceeding the mantle width. The arms had a single row of small suckers; the first six were sessile, and the rest were long and spindle-shaped with gelatinous stalks (Jereb et al., Reference Jereb, Roper, Norman and Finn2013). Only a few cirri were found in the present specimen. The web is deep, extending to the tips of the arms (Aldred et al., Reference Aldred, Nixon and Young1983). The total length of the studied animal was 27 cm. Other diagnostic features of this abyssopelagic species that we could observe were a very conspicuous shell with moderate and large, flared wings, with saddle length less than half-shell length; wings triangular from the lateral view.
In the family Opisthoteuthidae Verrill, 1896, four specimens of Opisthoteuthis grimaldii (Joubin 1903) have been reported: two specimens (male and likely female) in Guinea–Bissau waters, a male in waters off Western Sahara and a male in the waters of South Morocco (Appendix 1). Two specimens of Opisthoteuthis calypso Villanueva, Collins, Sánchez & Voss Reference Villanueva, Collins, Sánchez and Voss2002, one in waters off South Morocco and one off Western Sahara (Appendix 1), and one Opisthoteuthis massyae (Grimpe 1920) specimen (Figure 5) in Western Sahara waters (Appendix 1) were found. The latter three animals were tentatively identified by their mandibles and internal shells because the specimens were poorly preserved. The U-shaped shell and mandibles of the Western Sahara specimen were unequivocally identified as belonging to O. massyae, according to the description by Villanueva et al. (Reference Villanueva, Collins, Sánchez and Voss2002).
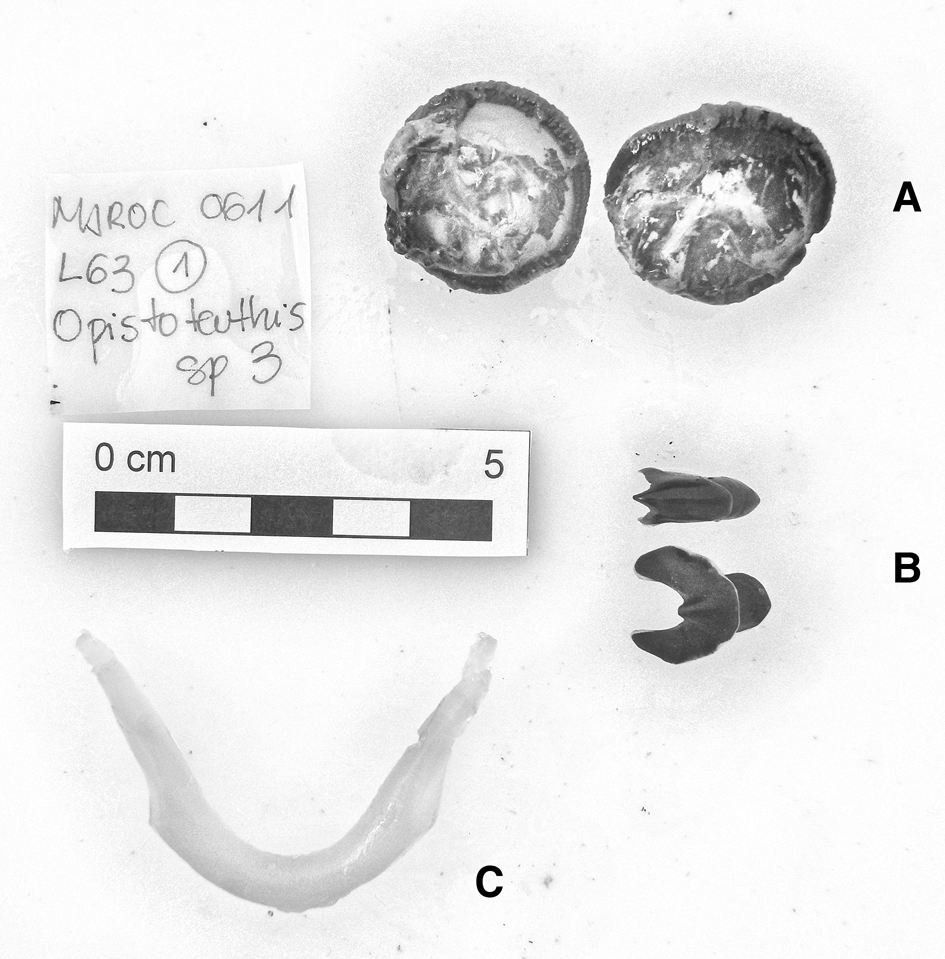
Fig. 5. Opisthoteuthis massyae (Grimpe, 1920) eyeballs (A), beaks (B) and shell (C). Maroc–0611 survey. Scale: each line, 1 cm. © Amanda Luna.
In Mauritanian waters, a female O. calypso was found. The specimen showed the diagnostic characteristics of the species according to Villanueva et al. (Reference Villanueva, Collins, Sánchez and Voss2002): arm sucker count in adults 47–58 (58 were counted); distal enlarged sucker field comprises 2-3 (3) contiguous suckers; the first cirrus usually occurs between suckers 1 and 2; maximum diameter of distal enlarged suckers does not exceed that of proximal enlarged suckers and the distal enlarged sucker field formula was IV.III.II.I.
Also, in Mauritanian waters, an O. massyae specimen was found. The individual was identified according to its markedly increased thickness of arms I in mature male; distal enlarged sucker field composed typically of 9–11 contiguous suckers (the specimen had 9 of them); maximum diameter of proximal enlarged suckers exceeds that of distal enlarged suckers; distal sucker enlargement absent on arms I, slight on arms II, greatest on arms III and IV and first cirri typically occur between suckers 3–4 or 4–5 (Villanueva et al., Reference Villanueva, Collins, Sánchez and Voss2002).
The three specimens were identified as O. grimaldii, one collected in South Morocco and two in Guinea–Bissau waters (Appendix 1). The diagnostic features of O. grimaldii were the absence of increased robustness of arms I in males; the presence of nine suckers in the distal enlarged sucker field of arms and the distal enlarged sucker diameter was not larger than the proximal one. Others were the distal enlarged sucker field formula (IV≥III≥I≥II), position of the first cirrus between suckers 2 and 3, arm sucker count (about 70) and subterminal fins (Villanueva et al., Reference Villanueva, Collins, Sánchez and Voss2002).
Analyses of another four Mauritanian specimens were performed using photographic material. One of them corresponded to O. massyae, another to O. calypso, and the other two specimens were not conclusive. Albeit, one of the not conclusive specimens had a conspicuous character attributed to only O. agassizii to date: the presence of pigment-free spots on the skin (Villanueva et al., Reference Villanueva, Collins, Sánchez and Voss2002). None among the other Atlantic species have this characteristic. Therefore, O. agassizii was retained in the final checklist for the CCLME area.
In the suborder Incirrata Grimpe, 1916, two specimens of Haliphron atlanticus Steenstrup, 1861, superfamily Argonautoidea Cantraine, 1841, were found. The first in Western Sahara and the second in Guinea–Bissau waters (Appendix 1). Identification of both animals was difficult because of damage, but the on-board identification and photographs taken during the surveys helped us. The transparent, gelatinous and nearly colourless appearance of the specimens (Figure 6) disappeared; the preserved specimens do not show these specific characters. The Guinea–Bissau specimen was female (about 9.0 cm ML) identified by the very wide mantle aperture and the eyes shape (Vecchione, personal communication). The Saharan specimen (about 7.0 cm ML) lacks the brachial crown, eyes and funnel structures, but it was identified by its gelatinous texture and comparison of the sucker structure and presence of a unique sucker row at the base of each arm with the Guinea–Bissau specimen. This species is characterized by the gelatinous and sac-shaped body; the suckers mostly in two series but grade to single series near the mouth, and the lack of enlarged arm suckers.
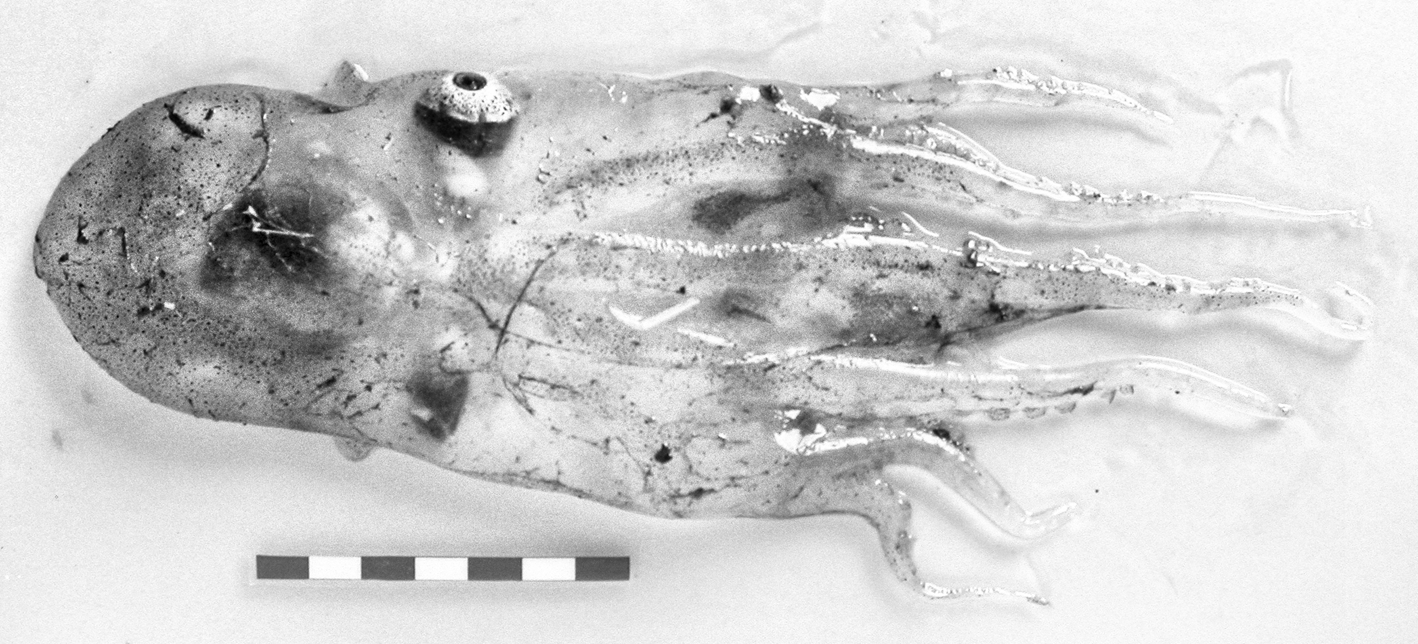
Fig. 6. Haliphron atlanticus Steenstrup, 1861. Dorsal view. Bissau–0810 survey. Scale: each line, 1 cm. © IEO.
In the family Bathypolypodidae Robson, 1932, eight specimens of Bathypolypus ergasticus (Fischer & Fischer, Reference Fischer and Fischer1892) were identified and measured: four in Moroccan waters (three males with 1.8, 5.5 and 9.0 cm ML and an undetermined specimen with 2.8 cm ML), another three specimens (two males and a female with 7.2, 4.5 and 8.1 cm ML) from waters of Guinea–Bissau and a male (9.2 cm ML) found among the specimens sampled from Mauritanian waters (Appendix 1). All the males were identified by the hectocotylus, which is very conspicuous in this species. The hectocotylus of B. ergasticus corresponds to the third right arm, which is shorter than the third left one and with 70–85 pairs of suckers. The ligula and calamus are open and pointed, although the latter may appear rounded, inconspicuous and lack suckers on the tip in juvenile specimens. The developed ligula (7% of hectocotylized arm length) has seven strong ridges, with a central rib and slightly developed scalloped walls. The spermatophore groove is strongly developed in the largest specimens. The medium-sized specimens have pointy and open-pointed calamus and ligula, with underdeveloped walls and spermatophoric groove. The undetermined specimen was identified based on eight lamellae in the outer demibranches of the gills and a UU-funnel organ composed of almost square-shaped pads (Muus, Reference Muus2002). The males had some autotomized arms, with subsequent regeneration evidence. Some diagnostic features found in the specimens were as follows: ink sac absent; not a very firm muscular body completely covered with small chromatophores, which gives it a purplish colour after fixation; mantle sac-like, as long as wide, smooth, with a wide palial aperture reaching to the back of the eyes, which are not very prominent and have a dark halo around them but no ocular papillae or warts; head narrower than the mantle, with a deep nuchal constriction; elongate triangular funnel, only separated from the body in its most distal region; elongated and thin subequal arms (77–87% ML), and round, small and quite separated biserial suckers (about 200 suckers per arm, in a zig-zag pattern, except the first pair placed in line; diameter, about 6% ML). Not greatly enlarged suckers. Well-developed web in the proximal half of the arms (about 25% of the longest arm length), that cause the arms to curve, was observed.
Several specimens of Bathypolypus valdiviae (Chun & Thiele, 1915) were identified (Figure 7): two females of B. valdiviae (3.5 and 3.4 cm ML) off Guinea–Bissau and a male (2.4 cm ML) in Mauritanian waters (Appendix 1). Some characteristic features of this short-armed big-eyed, warty species were observed such as a very wide mantle and presence of supraocular cirri. The specimens have subequal arms, which are rather thick basally and gradually narrow to fine extremities; a narrow pallial aperture and the funnel organ as a pair of widely separated V-shaped pads (Robson, Reference Robson1932). The warts of the 3.5 cm ML specimen were few and visible on the head, body and arms.

Fig. 7. Bathypolypus valdiviae (Thiele in Chun, 1915). Dorsal view. Bissau–0810 survey. Scale: each line, 1 cm. © IEO.
A specimen of the less common species Bathypolypus bairdii (Verrill, 1873), 2.2 cm ML, was identified from Mauritanian waters by the erectile pointed cirrus with adjacent smaller protuberances over each eye; papillated dorsal surface, especially in the anterodorsal region and square body, with a broad head and huge and prominent eyeballs (Muus, Reference Muus2002).
In the family Octopodidae, a specimen Octopus salutii Vérany, 1836 male with 6.0 cm ML was caught in Moroccan waters (Appendix 1). The diagnostic features of the species that we could observe were as follows: firm and muscular body, with a mantle short, broadly oval, and widest posteriorly; dorsal mantle covered with tiny irregular papillae; wide mantle aperture; head slightly narrower than mantle; a large papilla over each eye; arms long, subequal, tapering to narrow rounded tips; arms I shortest. Additionally, the specimen had a characteristic hectocotylus shape: right arm III of male hectocotylized shorter than the opposite arm, bearing 135–150 suckers; ligula long and slender, with deep groove and numerous fine transverse lamellae, margin slightly swelled; and calamus short (Mangold, Reference Mangold1998).
In the family Enteroctopodidae, four species were found: Muusoctopus januarii (Hoyle, 1885), Muusoctopus johnsonianus (Allcock, Strugnell, Ruggiero & Collins 2006), Muusoctopus levis (Hoyle, 1885) and Muusoctopus fuscus (Taki, Reference Taki1964).
A total of 15 specimens of M. januarii were found in the CCLME region (Appendix 1). A male was sampled from waters off South Morocco (8.5 cm ML); five other males (4.9, 4.2, 3.5, 2.2 and 2.1 cm ML) and a female (2.5 cm ML) were obtained from Western Sahara and two small males (1.5 and 2.0 cm) and six females (11, 3.5, 2.0, 2.0, 1.5 and 1.7 cm ML) from waters off Guinea–Bissau. These specimens were identified by the hectocotylus in the case of the males, which was consistent with the descriptions of Jereb et al. (Reference Jereb, Roper, Norman and Finn2013) and Guerra et al. (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014): third right arm hectocotylized almost twice shorter than the opposite, bearing ~80 suckers; ligula length from about 6–9% of the hectocotylized arm length, pointed and with a deep central groove, ~20 weakly developed transverse ridges within ligula groove; calamus small (from 15–25% of the hectocotylized arm), but well-defined, sharply pointed. Other diagnostic features observed in the specimens were as follows: large eyes; saccular and elongate mantle, smooth and devoid of sculpture, with a wide aperture; lack of ink sac; funnel robust, tapered, free for half its length; VV-shaped funnel organ, arms long and slender, cylindrical in cross-section, 3–4 times ML and attenuated towards the tips, becoming filiform; arms 1 and 2 markedly longer than 3 and 4 (1 = 2 > 3 = 4). Small and biserial suckers, with small infundibulum, in two moderately spaced rows directly from the mouth, with enlarged suckers absent, and longest unmodified arms with sucker count of ~180; gill with 7–8 lamellae per demibranch. After preservation, the dorsal surface of the specimens was pinkish grey to grey, with the ventral surface slightly paler (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014).
A juvenile female (4.0 cm ML) of M. johnsonianus was caught in Senegalese waters (Appendix 1). The diagnostic characteristics found in the specimen were consistent with the descriptions of Allcock et al. (Reference Allcock, Strugnell, Ruggiero and Collins2006) and Strugnell et al. (Reference Strugnell, Voight, Collins and Allcock2009): integument smooth; body and arms muscular, very soft; ink sac absent; a mantle slightly ovoid, with head slightly narrower than mantle; funnel short to moderate length, gently tapered; funnel organ W-shaped; arms long (~3.5 times ML) and subequal (2.1.3.4), with small biserial suckers closely set; anal flaps absent, and gills with 8–11 lamellae per demibranch (the specimen had 8). The preserved specimens have pale colouration and smooth skin (Allcock et al., Reference Allcock, Strugnell, Ruggiero and Collins2006). This was not observed due to preservation methods.
Two juvenile male specimens of M. levis (Figure 8) were sampled from Guinea–Bissau waters (Appendix 1). The specimens showed the characteristics of the species described in Robson (Reference Robson1932): smooth skin, firm and muscular body; head narrower than the saccular or ovoid body; prominent eyes; half-open mantle aperture; ink sac absent; arms short, small suckers (6–8% ML) without a discontinuous increase in size; web 43–33% of the longest arms; small funnel with a short free portion and VV-shaped funnel organ; eight filaments in each demibranch. The primordium of the hectocotylus was observed and fulfilled some particular features of the mature one: the hectocotylized arm is 71% of the longest arm; ligula length about 7% of the arm length; copulatory groove small but distinct, the laminae clearly marked and close-set; the calamus is very long, and its apex is more than half-way from the last sucker to the tip of the ligula, a very unusual feature.

Fig. 8. Muusoctopus levis (Hoyle, 1885). Dorsal view. Bissau–0810 survey. Scale: each line, 1 cm. © IEO.
In this study, two M. fuscus individuals were caught in Senegalese coast: a female and a male (both with 4 cm ML and about 19 cm TL). The diagnostic features observed in these specimens were coincident with Taki's (Reference Taki1964) description: a muscular body, with fairly well-developed musculature, and soft consistency; surface smooth throughout without any sculpture of integument; ink sac absent; mantle broadly ovoid, its width about 83% ML, widest at halfway of its length; mantle aperture very wide; head broad (61% ML), faintly constricted in front and behind; ocular cirri absent; eye orifice extremely large; slender and long arms, nearly circular in section; the longest arm was 82% of TL, and the arm formula is 2.1.3.4; arm suckers rather small with enlarged suckers absent; the diameter of the largest sucker is 6.1% ML. The male was identified by the hectocotylus: third right arm hectocotylized, 61% of longest arm length and 70% of the opposite mate, 40 pairs of suckers (37 were observed) in the ordinary part; conical ligula that represents 5% of hectocotylized arm length; copulatory groove rather shallow and narrow, pigmented; copulative lamina absent, with a faint roughness on both ridges of the copulatory groove; calamus pointed; very prominent flat seminal channel lacking chromatophores; gill with 7–11 leaflets in each demibranch; this species is characterized by its deep purplish colour, and dorsal and ventral surfaces are homochromatic, although the ventral side is partly lighter in colour.
Discussion
The cephalopods in the CCLME region
This paper completes an exhaustive review of cephalopod fauna present in the CCLME region. Currently, despite numerous surveys and previous studies (Hempel, Reference Hempel1982; Den Hartog, Reference Den Hartog1984; Van der Land, Reference Van der Land1987; Westphal et al., Reference Westphal, Freiwald, Hanebuth, Eisele, Gürs, Heindel, Michel and Reumont2007, Reference Westphal, Beuck, Braun, Freiwald, Hanebuth, Hetzinger, Klicpera, Kudrass, Lantzsch, Lundälv, Mateu-Vicens, Preto, Reumont, Schilling, Taviani and Wienberg2012), there is limited information on the deep-sea fauna and composition and structure of benthic communities of North-west Africa. Therefore, this is one of the least known regions of the world for deep fauna and benthic communities (Decker et al., Reference Decker, Griffiths, Prochazka, Ras, Whitfield, Decker, Griffiths, Prochazka, Ras and Whitfield2004).
Apart from some papers that refer to the biology of O. vulgaris (Caverivière et al., Reference Caverivière, Domain and Diallo1999; Diallo et al., Reference Diallo, Jouffre, Caverivière and Thiam2002), the biodiversity of Senegalese waters has not yet been studied. Here, 13 new species belonging to 10 families were identified. Adding to O. vulgaris, 14 cephalopod species from 10 families are the total number of cephalopods known in the waters off Senegal.
Species of special concern
Order Sepiida
No significant changes were found for the Sepiida species reported from the CCLME area, except for three species whose presence was previously doubted there: Sepia angulata Roeleveld, 1972; Sepia pharaonis Ehrenberg, 1831 and S. hieronis.
The distribution of S. angulata was noted as uncertain in the area because of the lack of information, and its presence is known only from cuttlebones in South Africa (Jereb & Roper, Reference Jereb and Roper2005; Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). Recently, Guerra et al. (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) indicated that this species is not present in the Eastern Central Atlantic. Therefore, S. angulata was removed from the final checklist for the area.
The species S. pharaonis was recorded in Guinea–Bissau waters by Fernández-Caballero (Reference Fernández-Caballero2014). However, the presence of this well-known species, which inhabits the Indian Ocean and western Pacific (Jereb & Roper, Reference Jereb and Roper2005) in the CCLME area is extremely doubtful. Moreover, Fernández-Caballero (Reference Fernández-Caballero2014) did not study sampled specimens but obtained the name from the records of Chinese fishing companies. Therefore, we concluded that the record of S. pharaonis in the Atlantic waters of Guinea–Bissau is attributable to a spurious identification and did not include this species in the checklist.
Sepia hieronis was recorded in CCLME waters in the report of Guinea–Morocco CCLME–1205 survey (Krakstad et al., Reference Krakstad, Michalsen, Høines, Alvheim, Zaera, Olsen and García–Isarch2012). Currently, this species is distributed from Namibia to Kenya (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). Therefore, the record of Krakstad et al. (Reference Krakstad, Michalsen, Høines, Alvheim, Zaera, Olsen and García–Isarch2012), made on board, is very unlikely. Unfortunately, we cannot study these specimens because most of them were not sampled for laboratory analysis. The few S. hieronis specimens sampled during the survey were lost during the transfer. It is necessary to find more material to confirm the expansion of its distribution. Therefore, S. hieronis was removed from the final checklist for the CCLME.
Order Sepiolida
In the family Sepiolidae, the presence of a species belonging to Rossiinae, A. mastigophora, in CCLME waters remained uncertain until now (Jereb & Roper, Reference Jereb and Roper2005; Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). Thus, two specimens of this species were identified for the first time in Western Sahara waters. The biology of this species is poorly known because of the few available records and the current low interest in fisheries (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). Its distribution in Eastern Africa was from Guinea to the Cape of Good Hope, up to a depth of ~640 m. Thus, the northern distribution range of A. mastigophora has been expanded off Western Sahara.
In the subfamily Sepiolinae, S. atlantica is distributed in the North-east Atlantic Ocean, from Iceland and Norway to North-west Africa (Morocco), and its southern limit was unknown (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). The specimens of this species collected in Western Saharan waters expanded its southern distribution limits off Western Sahara.
A species of the subfamily Heteroteuthinae Appellöf, 1898, Heteroteuthis dagamensis Robson, Reference Robson1924, was recorded off the western coast of Africa by Jereb & Roper (Reference Jereb and Roper2005), so this species was included in the list of cephalopods for Mauritanian waters by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). This species (as Heteroteuthis hawaiiensis var. dagamensis Robson, Reference Robson1924) was found in the Indian Ocean off South Africa (Robson, Reference Robson1924), but no specimens have been obtained from the CCLME waters to date. More recently, Rotermund & Guerrero-Kommritz (Reference Rotermund and Guerrero-Kommritz2010) clarified the taxonomy and biogeography of the genus Heteroteuthis Gray, 1849 in the Atlantic. They showed that H. dagamensis was distributed in only South Atlantic waters, and not in the CCLME region where only Heteroteuthis dispar (Rüppell, 1844) is present. Recently, H. dagamensis was found in the Gulf of Mexico and South Atlantic by Judkins et al. (Reference Judkins, Vecchione, Cook and Sutton2016). After, Braid & Bolstad (Reference Braid and Bolstad2019) found it in New Zealand, and Taite et al. (Reference Taite, Vecchione, Fennell and Allcock2020) in North Atlantic waters. Thus, although the presence of H. dagamensis in the area cannot be ruled out with the present data, new molecular or morphological data from the CCLME area are necessary to assess this question. Cautiously, the species was removed from the final checklist.
Superfamily Bathyteuthoidea
Chtenopteryx sicula has been reported in Western Sahara waters. This species was previously reported by Guerra et al. (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) in the CCLME area in waters off Morocco, Mauritania, Canary Islands, Guinea, Cape Verde and Azores. The overlap of the Canaries comb-finned squid Chtenopteryx canariensis Salcedo-Vargas & Guerrero-Kommritz, Reference Salcedo-Vargas and Guerrero-Kommritz2000, with the Sicilian comb-finned squid C. sicula in the studied area is clear. Chtenopteryx canariensis has a tropical Eastern Central Atlantic distribution from the Canary Islands to the equator, and is found at a depth of 1000 m (Salcedo-Vargas & Guerrero-Kommritz, Reference Salcedo-Vargas and Guerrero-Kommritz2000; Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014); C. sicula is a tropical-subtropical species that inhabits Eastern Atlantic waters from the Bay of Biscay to South Africa (36°S), in addition to the Mediterranean Sea, to a depth of 3000 m (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). However, it had an indisputable diagnostic character, the large photophores on the ventral surface of eyeballs, present in C. sicula but absent in C. canariensis specimens (Salcedo-Vargas & Guerrero-Kommritz, Reference Salcedo-Vargas and Guerrero-Kommritz2000; Escánez et al., Reference Escánez, González and Guerra2012, Reference Escánez, Roura, Riera, González and Guerra2018; Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). However, the presence of undescribed species in this genus is known (Young & Vecchione Reference Young and Vecchione2010) and supported by Braid & Bolstad (Reference Braid and Bolstad2019), which indicates that both families within Bathyteuthoidea are in need of revision, using integrative taxonomy.
Bathyteuthis abyssicola was found in Moroccan waters. Within this family, until now three species were recognized: B. abyssicola, a cosmopolitan species; Bathyteuthis bacidifera Roper, Reference Roper1968, known from the Eastern equatorial Pacific, and Bathyteuthis berryi Roper, Reference Roper1968, from the Eastern North Pacific (Roper, Reference Roper1968, Reference Roper1969; Jereb & Roper, Reference Jereb and Roper2010). Albeit, the taxonomy of the family is still unclear. Vecchione et al. (Reference Vecchione, Young and Piatkowski2010a) studied Bathyteuthis sp. A (cf. B. berryi Roper, Reference Roper1968), which is morphologically similar to B. berryi, and they pointed out that it is probably a new species inhabiting the North Atlantic. Bush et al. (Reference Bush, Hoving, Huffard, Robinson and Zeidberg2012) found a brooding female B. berryi and a male B. bacidifera in the Monterey Submarine Canyon in California. Using morphological analysis and DNA sequencing (COI, 16S), Judkins et al. (Reference Judkins, Lindgren, Villanueva, Clark and Vecchione2019) have described and named three new species from the North Atlantic Ocean: Bathyteuthis inopinata Judkins, Lindgren, Villanueva, Clark & Vecchione Reference Judkins, Lindgren, Villanueva, Clark and Vecchione2019, which corresponds to Bathyteuthis sp. A of Vecchione et al. (Reference Vecchione, Young and Piatkowski2010a) and Shea et al. (Reference Shea, Judkins, Staudinger, Dimkovikj, Lindgren and Vecchione2017), and probably B. abyssicola of Vecchione & Pohle (Reference Vecchione and Pohle2002); Bathyteuthis devoleii Judkins, Lindgren, Villanueva, Clark & Vecchione Reference Judkins, Lindgren, Villanueva, Clark and Vecchione2019 and Bathyteuthis numerosa Judkins, Lindgren, Villanueva, Clark & Vecchione Reference Judkins, Lindgren, Villanueva, Clark and Vecchione2019. Judkins et al. (Reference Judkins, Lindgren, Villanueva, Clark and Vecchione2019) did not describe the gladius and mandibles of the new species; consequently, we could not confirm that our specimen corresponds to any of them. Anyway, B. abyssicola was reported in Mauritanian waters by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017) because of its circumglobal distribution (Jereb & Roper, Reference Jereb and Roper2010). However, the most recent review of Atlantic cephalopods (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) showed that this cosmopolitan species is more frequently found in the Southern Ocean and productive waters of the Eastern Pacific, Atlantic and Indian Oceans. The fact that this species has also been reported in the Mediterranean Sea (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) could indicate the presence of B. abyssicola farther to the north, in CCLME waters. The identification of this species in Moroccan waters could confirm this fact.
Order Oegopsida
The peacock cranchiid squid, T. pavo is widely distributed in the Central North Atlantic Ocean from 59.98°N to the Southern Subtropical Convergence, and its distribution may be extended to the western Indian Ocean in the area of the Agulhas Current (Voss et al., Reference Voss, Stephen and Dong1992; Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014; Young, Reference Young2014). It was found in Mauritanian waters by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017), and, in this study, new specimens were found off Senegal.
Cycloteuthis akimushkini Filippova, 1968 was reported in Mauritanian waters by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). This species has been considered as a synonym of Cycloteuthis sirventi Joubin, 1919 (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014; ToL, 2019). The description of C. akimushkini was based on a very large specimen, and apparent differences between this species and C. sirventi may be due to size effects alone (ToL, 2019). Therefore, the species found by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017) should be C. sirventi. The presence of Discoteuthis laciniosa Young & Roper, 1969, from the same family was considered as uncertain in Mauritanian waters by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). Nevertheless, this species was reported in the Eastern Atlantic off West Africa, Madeira and Cabo Verde Islands to Mauritania by Guerra et al. (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014).
An A. (P.) redfieldi was first identified in Western Sahara waters. This species has been previously reported in Guinea–Bissau and South African waters in the Eastern Atlantic and off Senegal (Lu & Clarke, Reference Lu and Clarke1975), and it inhabits waters of 50 to ~720 m in depth (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). The northern limit of A. (P.) redfieldi is in Nova Scotia (Vecchione & Pohle, Reference Vecchione and Pohle2002).
The A. (H.) siedleckyi specimens identified in Moroccan waters represent a relevant contribution to its distribution range. The species was distributed in the South-east Atlantic, from the Schmitt–Ott Seamount to south-west from the Cape of Good Hope. Its known distribution did not exceed 34°S. Our knowledge of the known geographic distribution ranges of A. (H.) siedleckyi (Lipinski, Reference Lipinski1983; Sajikumar et al., Reference Sajikumar, Lipinski, Venkatesan, Sasikumar and Mohamed2018) have been expanded to western to 16°N, placing this species off Western Sahara, inside CCLME waters.
The presence of A. (A.) veranyi in Moroccan and Western Sahara waters, as well as Guinea–Bissau, Senegal and Mauritania coasts, is consistent with previous publications. The species has been reported in Guinean, Guinea–Bissau, Gambian, Senegalese, Mauritanian, Madeira and Mediterranean waters but not in Moroccan waters (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014; Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017).
Stigmatoteuthis arcturi has been previously reported in the North Atlantic Ocean, from Gibraltar to 45°S, and in the western Atlantic, from Nova Scotia to the Gulf of Mexico and Brazil (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). A specimen of Histioteuthis dofleini (Pfeffer, 1912) (synonymized name of Stigmatoteuthis dofleini Pfeffer, 1912) was mentioned by Gomes-Pereira et al. (Reference Gomes-Pereira, Gonçalves and Clarke2016), nowadays considered S. arcturi, off Azores Islands. The data presented here complete the southern distribution of this species that has a poorly understood biology and is not of interest for fisheries (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014).
Jereb & Roper (Reference Jereb and Roper2010) indicated that the L. grimaldii occurrence in Moroccan waters is probable, and Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017) reported its presence in the Mauritanian coast. Finally, the known distribution of L. grimaldii is the North-east Atlantic, from Ireland to Spain, and the Azores, Madeira and Canary Islands and eastern South Atlantic (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014; Escánez et al., Reference Escánez, Guerra, Rocha and Lozano-Soldevilla2017). Therefore, its presence in Moroccan waters was confirmed. The biology of this rarely captured squid is not well-known. Until recently, very few specimens have been studied: adults were found from the stomachs of predators (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014; Escánez et al., Reference Escánez, Guerra, Rocha and Lozano-Soldevilla2017); a juvenile with abortive tentacles and a few abortive suckers lacking horny rings was described by Clarke (Reference Clarke1964), and paralarvae (at least 1 cm ML) have been found (Young & Vecchione, Reference Young and Vecchione2016). The quasi-unequivocal diagnostic character of this species is dermal cushions covering the mantle. This character is shared with Pholidoteuthis adami Voss, Reference Voss1956 (Vecchione & Richard, Reference Vecchione and Richard2012). Both species have dermal cushions with similar histological structure; however, the lack of tentacles and the unique hook on arm II confirm the identification.
Magnoteuthis magna occurs throughout the tropical and warm temperate Atlantic, at least from 50°N, 27°W to 40°S, 26°W (Vecchione & Young, Reference Vecchione and Young2017). Guerra et al. (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) reported the species in Madeira and Azores Islands; Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017), in Mauritanian waters and Shea et al. (Reference Shea, Judkins, Staudinger, Dimkovikj, Lindgren and Vecchione2017), in New England waters. It has also been reported from the Indian Ocean by Nesis (Reference Nesis1987), but it could be a different species of Magnoteuthis (Vecchione & Young, Reference Vecchione and Young2017). In fact, another species, Mastigoteuthis inermis, described by Rancurel (Reference Rancurel1972) in the Ivory Coast waters, is currently considered as a synonym of M. magna (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). The geographic distribution of this species is better known after the captures in this study. In the case of both studied specimens, their characters are consistent with the original description by Joubin (Reference Joubin1920).
The existence of Mastigoteuthis flammea Chun, 1908 and Mastigoteuthis grimaldii (Joubin, 1895) in the Atlantic was reported by Nesis (Reference Nesis1987), but Vecchione & Young (Reference Vecchione and Young2014) placed them in synonymy with the valid species Mastigoteuthis agassizii Verrill, 1881. This species may have two forms: a north temperate/boreal, wrongly assigned to M. grimaldii and Mastigoteuthis schmidti Degner, 1925, and tropical (wrongly assigned to M. flammea), and they could not be separated with any certainty (Vecchione & Young, Reference Vecchione and Young2014). Mastigoteuthis flammea specimens were found in Mauritanian waters by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017); they should probably be reassigned as M. agassizii.
Several O. megaptera were found in South Moroccan waters, Western Sahara coast, Mauritania and Senegalese waters. This species has been recorded in Mauritania (Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017), Gulf of Guinea, Namibia and Central Atlantic waters (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). As has been indicated, three specimens were caught with trawls at 1367 to 1820 m, which exceed the 1100 m depth that is the maximum depth known for the species (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). Thus, knowledge of the northward expansion of the species’ documented geographic range and vertical distribution are reported.
In the family Onychoteuthidae, some remarkable species exist. Ancistroteuthis lichtensteinii is a species whose distribution appears very disjunctive because only a few specimens have been reported outside the Mediterranean in the scientific literature (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). Many onychoteuthid species distributions remain poorly understood, and actual absence from a region cannot be concluded from the absence of local records (Bolstad et al., Reference Bolstad, Braid, Strugnell, Lindgren, Lischka, Kubodera, Laptikhovsky and Roura2018). For example, Bolstad (Reference Bolstad2010) studied Onychoteuthidae from the Central and Southern Atlantic and did not include this species. It is known from the northern Mid-Atlantic Ridge (Vecchione et al., Reference Vecchione, Young and Piatkowski2010a). And, in the Eastern Atlantic, A. lichtensteinii has been reported from North-west Spanish waters, Sahara Bank, Mauritania and Angola (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014; Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). This species presumably could be found in the entire CCLME area. In the present study, specimens of this species were found in Moroccan, Western Sahara and Guinea–Bissau coast. According to Bolstad (Reference Bolstad2010), this species is distributed in North Atlantic waters, primarily 20–60°N, including the Mediterranean Sea, between the surface and 800 m. Therefore, the specimens are within the geographic range, except the Guinea–Bissau record that expands its distribution southwards. This genus has been considered monotypic since its description; however, the presence of a second species in Central Atlantic waters (A. lichtensteinii Type B) has been suggested by different authors (Kubodera et al., Reference Kubodera, Piatkowski, Okutani and Clarke1998; Vecchione et al., Reference Vecchione, Young and Tsuchiya2010b). About the O. robsoni specimen found, the known distribution of this species is subtropical to sub-Antarctic waters in the southern hemisphere (generally between 20° and 50°S), and in the Gulf of Mexico. The type locality is somewhere off Angola waters (Bolstad, Reference Bolstad2010). In the present study, the record of the specimen expanded the known geographic distribution range to Guinea–Bissau waters (Kubodera et al., Reference Kubodera, Piatkowski, Okutani and Clarke1998; Bolstad, Reference Bolstad2010). The observed characters of the present specimen were consistent with the description provided by Bolstad (Reference Bolstad2010) who remarked that O. robsoni juvenile specimens have not yet been described to date.
Finally, the presence of some oegopsid species is doubtful in the area. In the family Cranchiidae, Helicocranchia joubini (Voss, 1962) was reported to be probably present in Mauritanian waters (Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017) based on bibliographic data. However, the species was not recorded in the area by the most recent review of Atlantic cephalopods (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). This poorly known species is probably a synonym of Helicocranchia pfefferi Massy, 1907 (Jereb & Roper, Reference Jereb and Roper2010) also present in the CCLME zone (Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). For these reasons, H. joubini was considered a synonym of H. pfefferi and removed from the checklist.
Order Octopoda
In the order Octopoda, some families from the suborder Cirrata Grimpe, 1916 were remarkable. In the family Cirroteuthidae, the only species reported to date in the CCLME area is Cirrothauma magna (Hoyle 1885) (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). However, Guerra et al. (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) stated that C. murrayi might occur in the area because of its wide distribution range, although the assumed worldwide distribution of this species requires further review. Previously, Aldred et al. (Reference Aldred, Nixon and Young1983) recorded two specimens in Moroccan waters, which represents a unique record of this species in the area. Here, one C. murrayi specimen was found in South Moroccan waters although it was in a very bad condition, which caused difficulties in measurement procedures. Anyway, this record is a new contribution to information on this poorly known species and support its presence in CCLME waters.
Collins & Villanueva (Reference Collins and Villanueva2006) studied the family Opisthoteuthidae and reported three species in Eastern Atlantic waters: O. calypso, O. grimaldii and O. massyae. These species were also recorded in the North-west Atlantic (Villanueva et al., Reference Villanueva, Collins, Sánchez and Voss2002; Collins & Villanueva, Reference Collins and Villanueva2006) and Mauritanian waters (Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). In the present study, several specimens of O. grimaldii, O. calypso and O. massyae have been reported in the CCLME zone.
Based on the studies by Villanueva et al. (Reference Villanueva, Collins, Sánchez and Voss2002) and Collins & Villanueva (Reference Collins and Villanueva2006), the presence of O. agassizii in Mauritanian waters reported by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017) could be erroneous. Several authors have identified specimens from the North Atlantic as O. agassizii, but further studies assigned these records to O. calypso (Villanueva et al., Reference Villanueva, Collins, Sánchez and Voss2002; Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). As reported in the Results section, of the four examined Opisthoteuthis specimens, one was assigned to O. massyae, another O. calypso, whereas the analysis of the other two was not conclusive. Therefore, O. agassizii was retained in the final checklist for the CCLME area.
The geographic distribution of O. grimaldii (Joubin 1903) extends from the North-east Atlantic (west coast of the British Isles) to the South-east Atlantic (Namibia), passing through the Azores and off Cape Blanc (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). New specimens were identified as O. grimaldii in water off South Morocco and Guinea–Bissau.
The presence of several deep-water Bathypolyphus species (family Bathypolypodidae) in CCLME waters should be noted. Data given in this paper expanded the geographic distribution of B. ergasticus and B. valdiviae. The former species was reported from the North-east Atlantic to waters off Senegal (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). In the present study, its distribution expanded to Guinea–Bissau waters. Bathypolypus valdiviae (Figure 7) has been reported from the Agulhas Bank and adjacent areas in South Africa and off the Namibian coast (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). Until recently, this species was known only in the southern hemisphere (Muus, Reference Muus2002; Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). Specimens of this species have been found in Mauritanian waters (Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017), and, in the present study, new specimens of B. valdiviae were identified in Guinea–Bissau waters. As a result, the geographic distribution of the species is expanded to waters off Guinea–Bissau.
A specimen of the rare B. bairdii was identified in Mauritanian waters. It has been reported from north of the North Sea to southern parts of the Barents Sea, in the southern slopes of the Iceland–Greenland Ridge, from Labrador to Miami in West Atlantic waters (Muus, Reference Muus2002) and North-west Iberian waters in the East Atlantic (Pérez-Gándaras & Guerra, Reference Pérez-Gándaras and Guerra1978); it has been previously recorded in Mauritanian waters by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). Because of the records of this species in Mauritanian waters (this study; Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017), the replacement of B. bairdii with Bathypolypus sponsalis (P. Fischer & H. Fischer, Reference Fischer and Fischer1892) in the East Atlantic proposed by Roura et al. (Reference Roura, Guerra, González and Santiago-Pascual2010) is disproved. In consequence, we proposed that both species cohabit in this area.
Octopus salutii had been reported previously from Mediterranean and North-east Atlantic waters to the south of the Portuguese coast (Mangold, Reference Mangold1998; Jereb et al., Reference Jereb, Roper, Norman and Finn2013). The report of this species in Moroccan waters indicates that its geographic range should be expanded.
In the family Enteroctopodidae, great attention was paid to the poorly known species of M. januarii, M. johnsonianus, M. levis and M. fuscus. Muusoctopus januarii has been previously reported from waters off Mauritania (Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017), and it is possibly present in Namibian waters (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). The specimens found in waters off South Morocco and Guinea–Bissau exceeded the maximum known ML of 6.9 cm reported by Gleadall (Reference Gleadall2013). Also, the geographic distribution of M. januarii has been expanded to the north of the eastern coast of Africa, from Guinea–Bissau and passing through Western Sahara to Moroccan waters.
Muusoctopus johnsonianus has been recorded in the Atlantic coast of Europe between 49–59°N (Porcupine Seabight, Ireland, to Rockall Trough, UK) at a depth of 1800–2540 m (Allcock et al., Reference Allcock, Strugnell, Ruggiero and Collins2006). Now, the individuals caught in Senegalese waters extended its geographic range to West African waters.
Muusoctopus levis has been reported only from Australian waters (Heard Island; Robson, Reference Robson1932). The presence of M. levis in Atlantic waters expands the distribution range of this species, and it is the first time that M. levis has been reported from Atlantic waters and observed in the northern hemisphere. This is a poorly known species and its presence in Atlantic waters represents an extraordinary extension of its geographic distribution. Thus, the presence of specimens of M. levis should be sought between the two known locations to complete and understand its distribution. Deeper taxonomic studies are needed, both morphological and genetic, to clarify and check the correct identification of this species and determine its true range.
Muusoctopus fuscus has been recorded only in Kashima Nada (Japan; Taki, Reference Taki1964) and, recently, in Mauritanian waters (Rocha et al., Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). In this study, new M. fuscus individuals were caught in Senegalese waters. Although the characteristics of our specimens fit the previous descriptions of M. fuscus, it would be advisable to conduct molecular studies of these specimens and get additional samples to confirm their presence in the area. The two populations could co-exist through the Indian Ocean and the East African coast, which implies that the deep-sea octopod collections in these areas should be reviewed to check if more specimens of this species are present. The substantial geographic distance between these two regions warrants further investigation.
In the suborder Incirrata, a member of the superfamily Argonautoidea, H. atlanticus, was found. This is a very widely distributed cosmopolitan species that inhabits from tropical to high latitudes (Guerra et al., Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014). The species was also found in water off Mauritania by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017). However, the identity of these individuals must be considered carefully because the molecular evidence of Lima et al. (Reference Lima, Mendes, Veras, Leite and Lima2017) suggests that the name H. atlanticus might encompass two species, both of them cohabiting Atlantic waters. Thus, further morphological and molecular systematics studies using more specimens of this species are needed to confirm this hypothesis.
The presence of Tremoctopus gelatus Thomas, 1977, has been mentioned as uncertain in Mauritanian waters by Rocha et al. (Reference Rocha, Fernández-Gago, Ramil, Ramos, Ramos, Sanz and Ramil2017) because no specimens of this species were found by these authors. Moreover, Guerra et al. (Reference Guerra, González, Roeleveld, Jereb, Carpenter and De Angelis2014) do not mention the presence of this species in the CCLME area. Therefore, we considered that T. gelatus is not present in the area and removed it from the final checklist for cephalopod species in the CCLME.
Final considerations
If we consider the 63 cephalopod species studied in this article together with the updated checklist of 137 cephalopod species for the zone, we can conclude that the CCLME area has been revealed to have a wide and diverse cephalopod fauna. The global patterns of species richness (Rosa et al., Reference Rosa, Pissarra, Borges, Xavier, Gleadall, Golikov, Bello, Morais, Lishchenko, Roura, Judkins, Ibáñez, Piatkowski, Vecchione and Villanueva2019) indicate that the number of coastal cephalopods in the Atlantic is 95 species, which is the third most diverse ocean after the Pacific (213 cephalopod species) and Indian (146 species) Oceans. Clarke (Reference Clarke2006) reported 82 oceanic mid-water cephalopod species and 16 shelf and slope species in the eastern North Atlantic between 10° and 70°N. Vecchione et al. (Reference Vecchione, Young and Piatkowski2010a) reported 56 cephalopod species (mostly oceanic species) in the northern Mid-Atlantic Ridge. Shea et al. (Reference Shea, Judkins, Staudinger, Dimkovikj, Lindgren and Vecchione2017) reported 75 mid-water cephalopod species and 28 benthic species in the Bear Seamount (New England). Thus, the importance of these findings allowed us to provide new information on the diversity of cephalopod species in this scarcely studied zone. New technologies, including video exploration of mid-water and deep seas, could shed more light on global cephalopod biodiversity.
This article completes the taxonomic studies about the cephalopod fauna on the Atlantic coast of Africa. This is one of the projects performed in the area to evaluate the resources and reference the state of this ecosystem at the regional level (Ramos et al., Reference Ramos, Faraj, Balguerías, Belcaid, Burgos, Gómez, González, Hakim, Hernández, Manchih, Meiners, Ramil, Salmerón, Sanz and Settih2005; Hernández-González et al., Reference Hernández-González, Faraj, Balguerías, Belcaid, Burgos, Cansado, Fernández, González, Jiménez, Manchih, Meiners, Muñoz, Nuño, Presas, Ramos, Salmerón, Settih and Soto2006, Reference Hernández-González, Bouzouma, Burgos, Hernández and Cheikna2008; Hernández-González, Reference Hernández-González2007; García-Isarch et al., Reference García-Isarch, Burgos, Sobrino, Almeida, Barry, Nahada, Funny and Gómez2009; Ramos et al., Reference Ramos, Alcalá, Fernández, Fernández, González-Porto, López, Moya, Pascual, Presas, Puerto, Ramil, Salmerón, Sanz, Rey, Viscasillas, Abed, Baye, Ciré, Mohamed, Samba and Valy2010; Krakstad et al., Reference Krakstad, Michalsen, Alvheim, Zaera, Bagøien, Krafft, García-Isarch, Ramil and Van Waerebeek2011, Reference Krakstad, Michalsen, Høines, Alvheim, Zaera, Olsen and García–Isarch2012).
Acknowledgements
We are grateful to all our colleagues who participated in the different cruises for our access to a valuable cephalopod collection, and very especially to Ana Ramos for all her help and support. We would like to thank Ángel Guerra and Fernando Ángel Fernández-Álvarez for their help in the most difficult taxonomic identifications. We offer special thanks to IEO researchers Sebastián Jiménez, Lourdes Fernández, Eva Hernández, Carlos Hernández and Eva García-Isarch for their help and for providing us with basic information on the Maroc, Maurit and Bissau cruises, and Oxford Science Editing for their invaluable help in improving the language of the manuscript. We would like to make a special mention to Aurora Bartolomé for her priceless help with the detail photos. We also greatly appreciate the constructive and accurate comments of Michael Vecchione and the anonymous referees on the original manuscript. This is ECOAFRIK publication number 42. This article received no specific grant from any funding agency, commercial or not-for-profit sectors.
Appendix 1. Data of species of special concern studied by survey and station.















