Introduction
A fundamental concept in evolutionary biology is species phenotypic variation in response to divergences of biological and abiotic conditions or genetic flow interchange (Khan et al., Reference Khan, Miyan and Khan2013; Theis et al., Reference Theis, Ronco, Indermaus, Salzburger and Egger2014). These biotic and abiotic conditions exert strong pressures on organisms, being a mechanism for phenotypic variations that may favour their performance under local conditions (Franssen et al., Reference Franssen, Stewart and Schaefer2013; Fulton et al., Reference Fulton, Binning, Wainwright and Bellwood2013; Culumber & Tobler, Reference Culumber and Tobler2016). Thus, adaptation to a particular habitat may lead to specialization when reproductive isolation accumulates as a by-product of adaptive divergence, or when different mutations occur in geographically separate populations (Rundle & Nosil, Reference Rundle and Nosil2005; Khan et al., Reference Khan, Miyan and Khan2013). Morphological differences can be attributed to phenotypic plasticity that optimizes aptitudes in different habitats (Franssen et al., Reference Franssen, Stewart and Schaefer2013; Oufiero & Whitlow, Reference Oufiero and Whitlow2016). These morphological changes and reproductive success may increase survival, allowing the species to adapt to changeable environmental conditions (Pfennig et al., Reference Pfennig, Wund, Snell-Rood, Cruickshank, Schlichting and Moczek2010). Phenotypic plasticity is well documented in fish (see Wiens et al., Reference Wiens, Crispo and Chapman2014; Istead et al., Reference Istead, Yavno and Foxa2015; Oufiero & Whitlow, Reference Oufiero and Whitlow2016). Individuals belonging to a given species raised under different conditions may exhibit distinct morphological characteristics. Therefore, fish tend to differ with morphologies that give them some evolutionary advantage, aiming to maximize their suitability for a given habitat (Sukumara et al., Reference Sukumara, Gopalakrishnan, Sebastian, Vijayagopal, Nandakuma Rao, Raju, Ismail, Abdussamad, Asokan, Said Koya and Rohit2016).
It is widely accepted that species evolution is induced by the reduction of genetic flow, often due to the existence of physical barriers (Langerhans et al., Reference Langerhans, Layman, Langerhans and DeWitt2003; Culumber & Tobler, Reference Culumber and Tobler2016). According to Kawata (Reference Kawata2002), this restriction of genetic flow throughout the generations favours the reproductive isolation of the species leading to geographic variations. Thus, decreases or lack of direct interchange between specimens of different areas can result in differences in qualitative (morphological) and quantitative (meristic) characters. A lack of genetic flow is more likely among freshwater fish than marine fish, where there is no obvious physical barrier (Schluter, Reference Schluter2001). According to Mayr (Reference Mayr1954), evolution of marine species can be operated by a set of rules different from those of freshwater, given the difficulties to apply allopatric models of speciation. First, there are few opportunities for geographic isolation in a circumglobal aquatic environment like the ocean. Second, most marine organisms have a pelagic larval phase that has an enormous dispersion potential (Palumbi, Reference Palumbi1994; Mora & Sale, Reference Mora and Sale2002).
Anchoa januaria (Steindachner, 1879) is a small pelagic fish of the family Engraulidae that reaches a maximum size at ~8.5 cm total length (TL) with a diet composed mainly of zooplankton. It is found across coastal systems of the tropical and subtropical Brazilian coast (Nizinski & Munroe, Reference Nizinski, Munroe and Carpenter2002). This species reaches first maturity at 65 mm TL (Esper, Reference Esper1982) and occurs in low salinity water of semi-enclosed estuarine systems, migrating to the lower reaches of rivers to spawn (Silva & Araújo, Reference Silva and Araújo2000; Santos et al., Reference Santos, Araujo, Silva and Vasconcellos2007). Cervigón (Reference Cervigón1969), studying their geographic distribution and comparing meristic characters between specimens from Brazil's north-east region (8°S) and south-east region (24–25°S), proposed that specimens from these two areas belong to two distinct populations because of changes in the number of gill rakers. Other studies recorded changes in morphological characters of members of the family Engraulidae and suggested that environmental conditions are playing an important role that result in morphological divergence (Erdoğan et al., Reference Erdoğan, Turan and Koç2009; Xie, Reference Xie2012). Erdoğan et al. (Reference Erdoğan, Turan and Koç2009) found morphological divergence in Engraulis encrasicolus (Linnaeus, 1758) among the Black, Marmara and Aegean Seas, and suggested that environmental conditions contributed to morphological variation as there are differences in productivity and temperature between the three studied environments.
We compared morphometric and meristic characters of A. januaria between two coastal areas in the Tropical and Subtropical regions of Brazil. The aim was to assess morphological divergence between individuals from the two regions and to determine whether such differences result in two conspecific populations: (1) Sepetiba Bay and Guandu River that are located in the Tropical region (23°S); and (2) Paranaguá Bay and Itiberê River that are located in the Subtropical region (25°S). The tested hypothesis is that morphological divergence occurs between populations of these two regions as a consequence of different environmental conditions. The following questions were postulated: (1) Do the populations of A. januaria have heterogeneity in the morphometric and meristic characters between the two studied areas? (2) Is it possible to discriminate between populations along the Tropical/Subtropical Brazilian coasts?
Materials and methods
Study area and fish handling
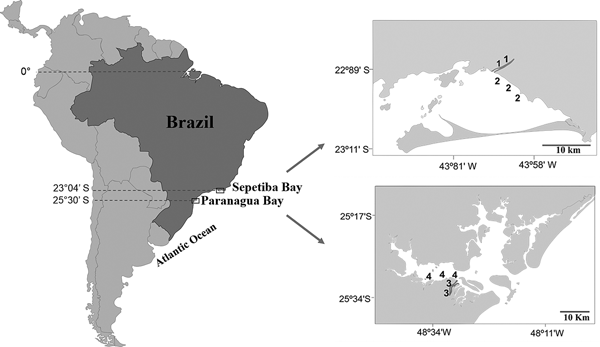
Sepetiba Bay (22°55′–23°49′S 43°35′–44°11′W) is one of the largest coastal ecosystems in the state of Rio de Janeiro (Figure 1), covering a wide range of habitats, including mangroves, sand beaches and small estuarine areas (Fiszman et al., Reference Fiszman, Pfeiffer and Lacerda1984; Leal Neto et al., Reference Leal Neto, Legey, González-Araya and Jablonski2006). The depth in most of the bay ranges from 2–12 m and its waters are rich in organic nutrients from continental drainage. The average temperature ranges from 20–25°C in the winter and 26–30°C in the summer. The average salinity is 30 (range: 28–33). The climate is tropical humid with annual precipitation between 1000 and 2100 mm. Rainfall peaks occur between December and January, whereas the driest period is between May and September. The bay supports a rich and diverse fish fauna, with a total of 148 recorded species (Araújo et al., Reference Araújo, Azevedo and Guedes2016). Despite its great ecological importance, an increased degradation is being caused by Rio de Janeiro industrial plants that bring into the bay effluents and pollutants via rivers and drainage channels (Molisani et al., Reference Molisani, Kjerfve, Silva and Lacerda2006; Cunha et al., Reference Cunha, Rocha, Geraldes, Pereira and Almeida2009; Araújo et al., Reference Araújo, Azevedo and Guedes2016). The Guandu River is 108.5 km long and is one of the most exploited systems in south-eastern Brazil because it supplies water for the municipality of Rio de Janeiro and several industrial plants in its lower reaches. Its lower reaches, mostly channelized, are next to a large industrial development, with muddy substrate and tidal influence. The riparian cover is composed mainly of grass.
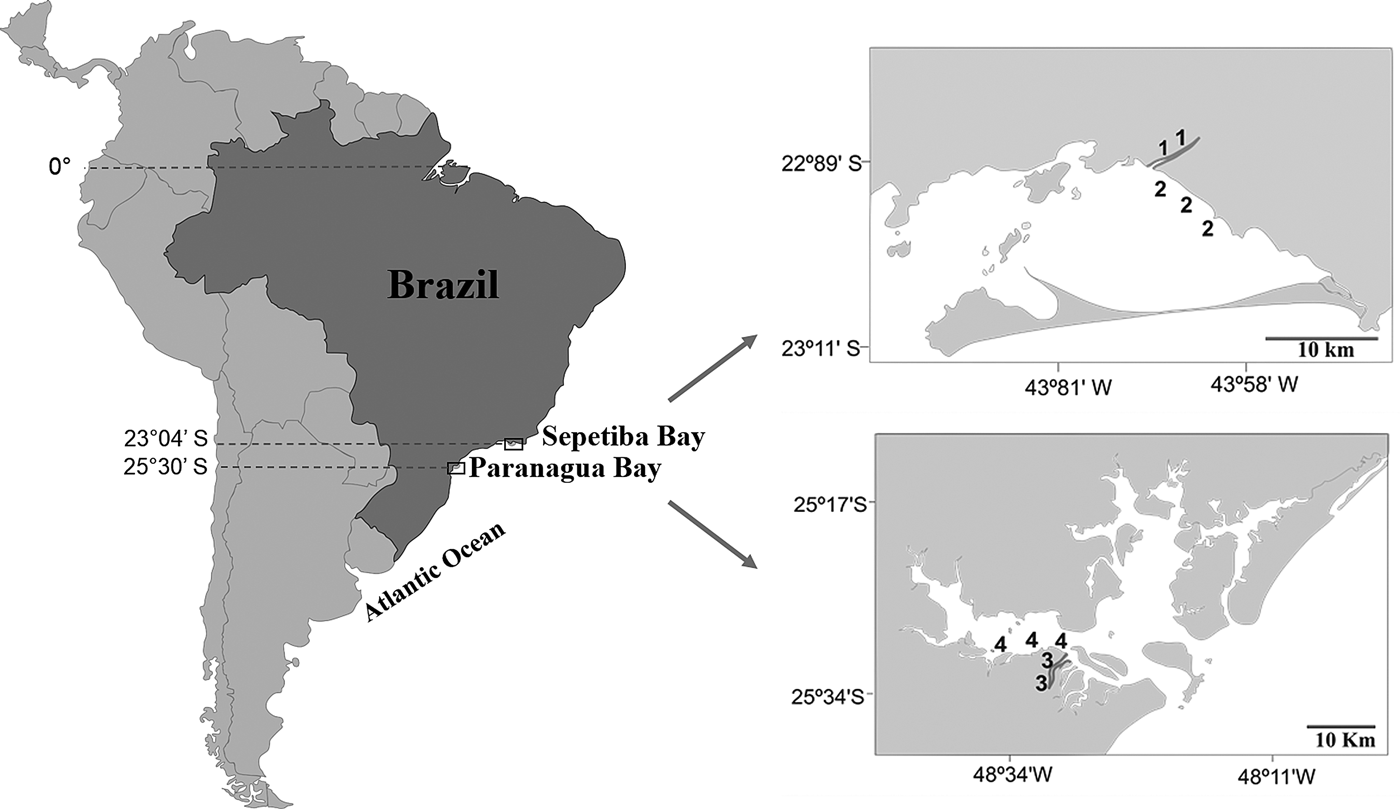
Fig. 1. Study area with indication of the two localities in the Tropical (1, Guandu River; 2, Sepetiba Bay) and Subtropical (3, Itiberê River; 4. Paranaguá Bay) regions.
The estuarine complex of Paranaguá (25°30′S 48°40′W) is located on the southern coast of Brazil (Figure 1) and encompasses a wide diversity of environments, including tidal rivers (Lana et al., Reference Lana, Marone, Lopes, Machado, Seeliger and Kjerfve2001). Average temperatures vary between 18–23°C in winter, and 24–28°C in summer, whereas the salinity ranges from 12–29 in summer and 20–34 in winter. The climate is subtropical, with annual rainfall of 2500 mm, with two well-defined seasons: rainy in the summer (October–March) and dry in the winter (April–September). Various anthropogenic activities in the bay shoreline contribute to environmental degradation, such as urban occupation, ports, fisheries, all of which affect the environmental quality of this estuarine system. The Itiberê River skirts the city of Paranaguá, separating Valadares Island from the continent. Its margins were originally mangrove swamps that were destroyed as a result of the expansion of the city (Caneparo, Reference Caneparo2000). Now only small mangrove forest fragments remain, the roots of which, along with the columns of many piers, are covered by encrusting communities.
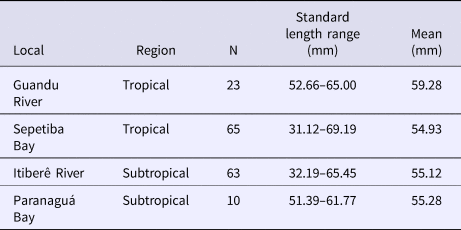
Fish from Sepetiba Bay and Guandu River were collected using beach seines and bottom trawls. Immediately after collection, all individuals were anaesthetized in benzocaine hydrochloride (50 mg l−1) and then fixed in a 10% formaldehyde-seawater solution. After 48 h, the specimens were transferred to 70% ethanol. All individuals were identified to species and morphometric and meristic measurements were taken on the left side of each individual. Fish from Paranaguá Bay and Itiberê River were collected by similar gears and deposited in the Museu de Zoologia (Fish Collection), Universidade de São Paulo (Table 1).
Table 1. Number of individuals (N) and size range in the four localities
Morphometric and meristic measurements
Morphometric and meristic measurements were taken on the left side of each individual. We used a digital calliper for the measurements with 0.05 mm precision. The following characters (Figure 2) were measured/counted under stereomicroscopy: (1) total length (TL), distance between the tip of the snout and the posterior end of the caudal fin; (2) standard length (SL), distance between the tip of the snout and the end of the hypural plate; (3) head length (HL), distance from the snout to the most distal part of the opercula; (4) eye diameter (ED), horizontal distance between the anterior and posterior margins of the orbit; (5) upper maxilla length (UML), distance between the pre-maxillary symphysis and the maxillary end; (6) pectoral fin length (PFL), distance from the origin of the pectoral fin to the end of the largest ray; (7) mouth length (ML), distance between the anterior tip of the upper maxilla to the posterior part of the mouth opening; (8) pre-pectoral distance (PPD), distance from the tip of the snout to the basal region of the pectoral fin; (9) pre-pelvic distance (PPeD), distance from the tip of the snout to the basal region of the pelvic fin; (10) pre-dorsal distance (PDD), distance from the tip of the snout to the base of the first ray of the dorsal fin; (11) pre-anal distance (PAD), distance from the tip of the snout to the base of the first ray of the anal fin; (12) anus-anal fin distance (AAFD), distance from the anus to the base of the first ray of the anal fin; (13) body height (BH), the highest body height; (14) head height (HH), the highest head height; (15) peduncle (P), the highest height in the peduncle (fish body between the anal and caudal fin); (16) NPR, number of pectoral fin rays; (17) NPeR, number of pelvic fin rays; (18) NDR, number of dorsal fin rays; (19) NAR, number of anal fin rays; (20) NRU, number of rakers of the first upper branchial arch; and (21) NRL, number of rakers of the first lower branchial arch.
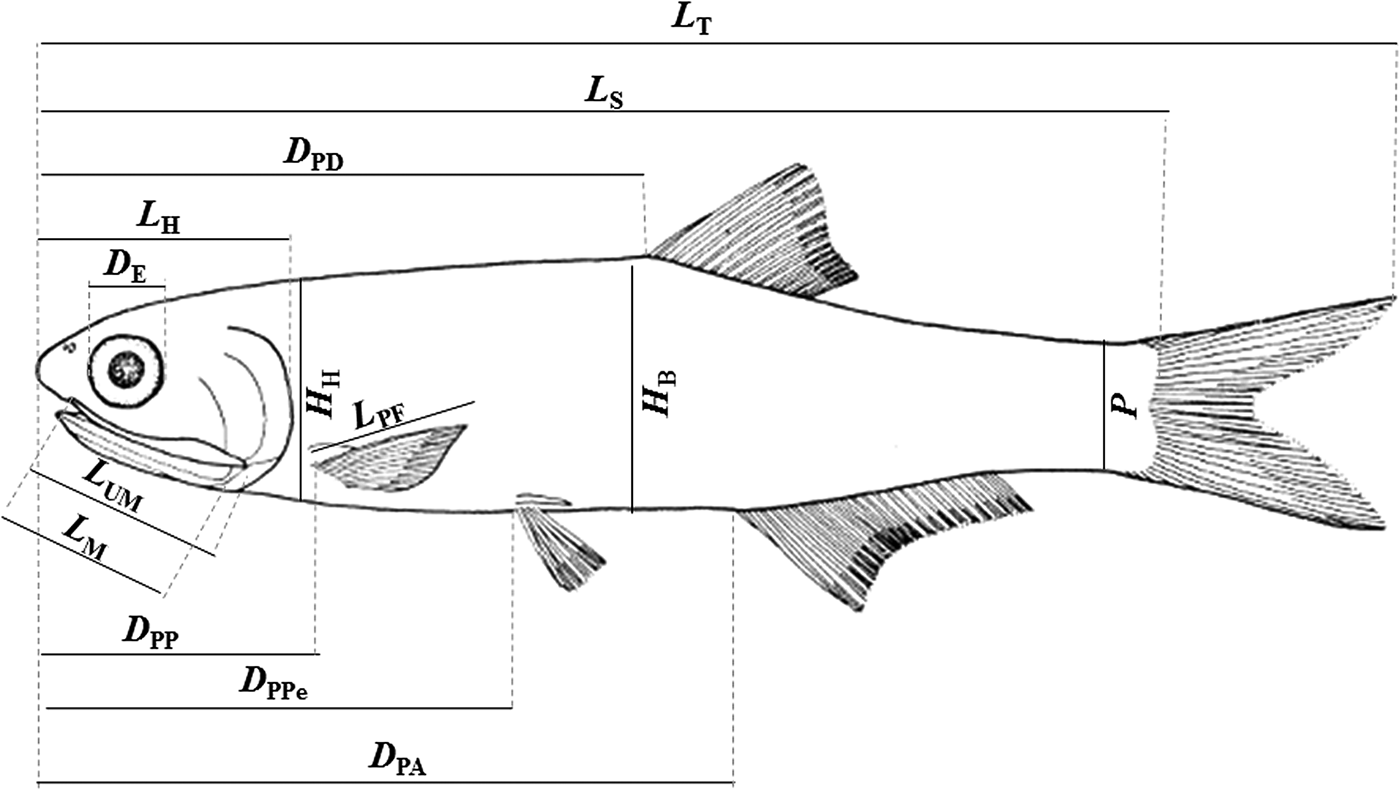
Fig. 2. Schematic diagram of Anchoa januaria with indication of the morphometric measurements. L T, total length; L S, standard length; L H, head length; D E, eye diameter; L UM, upper maxilla length; L PF, pectoral fin length; L M, mouth length; D PP, pre-pectoral distance; D PPe, pre-pelvic distance; D PD, pre-dorsal distance; D PA, pre-anal distance; H B, body height; H H, head height; P, peduncle.
Data analyses
A linear regression analysis was applied to the continuous quantitative variables (body measurements) against the standard length, body weight and head length. The meristic and morphometric characters were compared between the two regions using a non-parametric Mann–Whitney U test, whereas the regression coefficients of morphometric relationships (slopes) were compared using analysis of covariance (ANCOVA). Data from the Guandu River and Sepetiba Bay were pooled and treated as the information from the Tropical region, whereas the combined data from the Itiberê River and Paranaguá Bay were treated as information from the Subtropical region.
The raw morphometric data were logarithmized and submitted to the Aitchinson (Reference Aitchinson1986) transformation to remove the size effect (ontogenetic effect) using the following equation: Y ij = log x ij-1/p ∑p ilog x ij, where p denotes the number of characters being considered and x ij represents the value for the i th individual and j th character. The Aitchinson transformation (Reference Aitchinson1986) is more suitable than the residues method to remove the size effect (Reist, Reference Reist1986; Fleming et al., Reference Fleming, Jonsson and Gross1994) because first, each individual is independently scaled, and second, it is applied as a general measure of size based on all variables.
Analysis of variance (ANOVA) followed by a posterior Bonferroni test on the log-transformed and independent size data at 0.01% significance level were applied to compare the means of the specimens' characters among the four localities (Guandu River, Sepetiba Bay, Itiberê River and Paranaguá Bay). In addition, a principal component analysis (PCA), based on the variance-covariance matrix of the morphometric characters were applied to detect eventual morphometric patterns among the localities. According to Neff & Marcus (Reference Neff and Marcus1980), this procedure, a priori, is used to treat different samples as a homogeneous group, and there is no need to define the groups. Finally, a canonical discriminant analysis was used to assess the existence of isolated groups and how the groups relate to each other.
Results
Morphometric characters
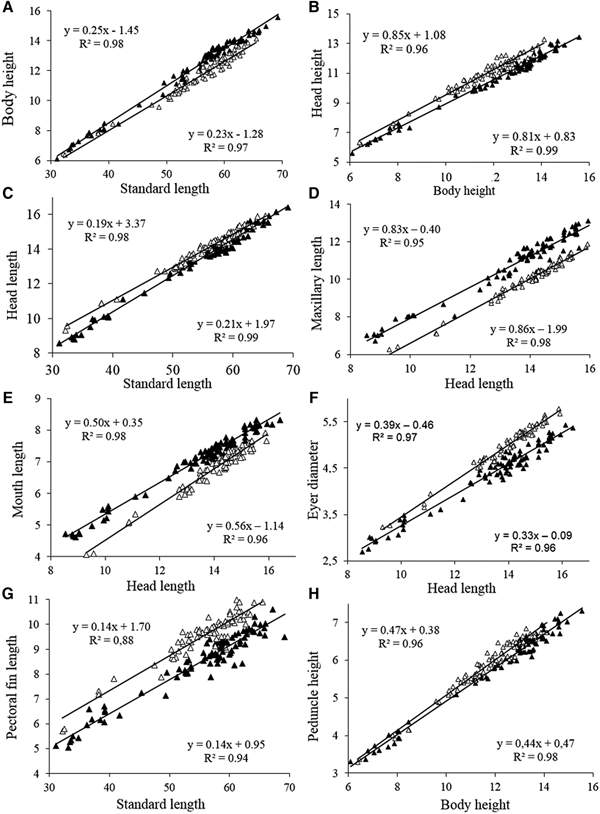
Fish size (in standard length) did not differ significantly between the two areas (Mann-Whitney, Z = 0.62, P = 0.53) ranging from 31.1–69.2 mm SL in the Tropical region and 32.1–65.5 mm SL in the Subtropical region. The body height was significantly higher (Z = 3.93, P = 0.0003) in the fishes of the Tropical region compared with those of the Subtropical region, with differences being more evident in individuals more than 50 mm SL (Figure 3A). The angular coefficients (slopes) of the regressions between body height and standard length differed significantly between the two regions according to ANCOVA (F 1,158 = 8.07, P = 0.005). Regarding the relationship between the height of the head and the height of the body, we found that the specimens from the Subtropical region had a significantly (ANCOVA, F 1,158 = 278.8, P = 0.0001) higher head height (Figure 3B) compared with those from the Tropical region. The angular coefficients (slope) of the regressions did not differ significantly (F = 2.6, P = 0.11). The head length did not differ between individuals of the two regions, especially in sizes larger than 50 mm SL (Figure 3C).

Fig. 3. Scatterplots of morphometric characters (mm) of A. januaria from the Tropical (black triangles) and the Subtropical (white triangles) regions.
The relationship between maxillary length and head length indicated that specimens from the Tropical region had a comparatively higher maxillary length (F 1,158 = 2023, P = 0.00001) with the angular coefficients of the regressions not differing significantly between the two regions (F = 2.3, P = 0.13) (Figure 3D). The mouth length was significantly larger for specimens from the Tropical region compared with those from the Subtropical region (Z = 3.67, P = 0.0002). In addition, the relationship between mouth length and head length, showed that specimens from the Tropical region had longer mouth length (F 1,158 = 554, P = 0.0001) with the slopes (regression coefficients) differing significantly between the two regions (F = 18.9, P = 0.0001) (Figure 3E).
Specimens from the Subtropical region had comparatively larger eye diameter than those from the Tropical region (Z = 6.64, F = 0.0001), with a trend for higher differences in individuals with larger head lengths, since the slopes differed significantly (F = 22.9, P = 0.0001) between the two regions (Figure 3F). The relationship between the length of the pectoral fin and the standard length showed that the specimens from the Subtropical region had larger pectoral fins compared with those from the Tropical region, with no significant difference between the slopes (Slope F = 0.5; P = 0.46) (Figure 3G). The caudal peduncle height did not differ significantly between specimens of the two regions (Z = 2.0, P = 0.06) and most samples of both regions overlap in the plots of the relationship between the height of the caudal peduncle and the body height (Figure 3H).
Morphometric characters independent of size
Six of the 15 morphometric characters independent of size differed significantly in the individuals among the four localities/groups (Sepetiba Bay, Guandu River, Paranaguá Bay and Itiberê River) according to the Mann–Whitney test: head height, head length, body height, maxillary length, eye diameter and pectoral fin length. However, the pre-pectoral distance (Z = 0.5, P = 0.61), pre-dorsal distance (Z = 1.18, P = 0.23), pre-pelvic distance (Z = 0.7, P = 0.48) and anus-anal fin distance (Z = 1.1, P = 0.26) did not differ significantly in individuals among the four localities.
The most important variables that contributed to the localities discrimination were the body height (Z = 3.9, P = 0.0001), maxillary length (Z = 5.9, P = 0.0001), pectoral fin length (Z = 6.2, P = 0.0001), eye diameter (Z = 6.6, P = 0.001) and mouth length (Z = 6.6, P = 0.0001).
Head height had lower values for specimens from the Guandu River in relation to the other three areas, that did not differ among them (P < 0.001; Figure 4A). Specimens of the Guandu River and Paranaguá Bay did not differ significantly in head length, although individuals from the Sepetiba Bay have significantly larger head length than those from the Itiberê River (P < 0.001; Figure 4B).
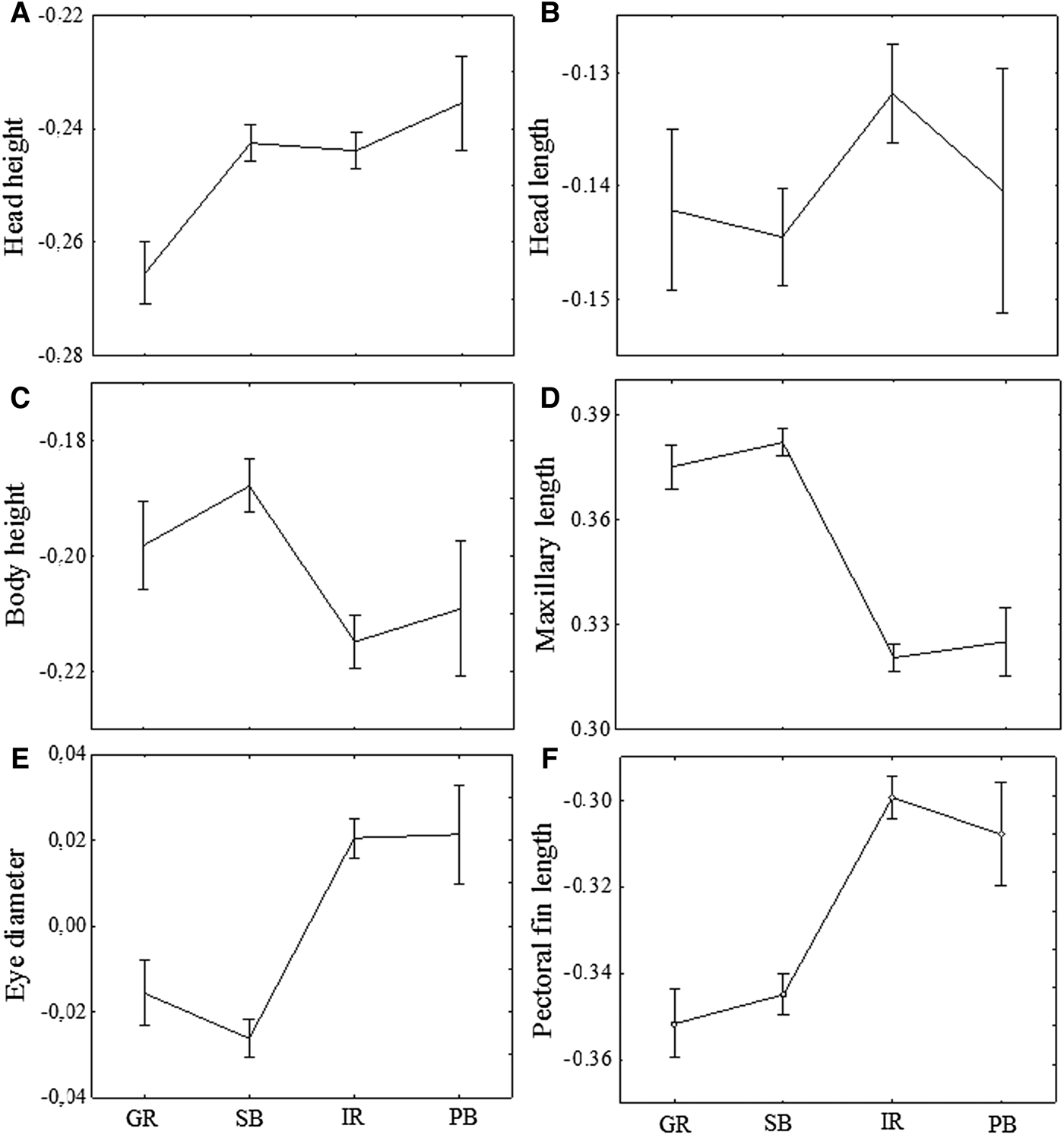
Fig. 4. Means and standard errors (vertical lines) for morphometric characters of A. januaria after the Aitchinson transformation of log-transformed data. GR, Guandu River; SB, Sepetiba Bay; IR, Itiberê River; PB, Paranaguá Bay.
The body height and the upper maxillary length were larger in specimens from the Sepetiba Bay and Guandu River compared with those from Paranaguá Bay and Itiberê River (P < 0.001). In addition, no significant differences in these two characters were found between the specimens from Guandu River and Sepetiba Bay, and between the specimens from Itiberê River and Paranagua Bay (Figure 4C, 4D).
The eye diameter and the pectoral fin length were larger in specimens from the Itiberê River and Paranaguá Bay compared with those from the Guandu River and Sepetiba Bay (P < 0.001; Figure 4E, 4F). Similarly, no significant differences in these two characters were found between the specimens from Guandu River and Sepetiba Bay, and between the specimens from Itiberê River and Paranagua Bay (Figure 4E, 4F).
Meristic characters
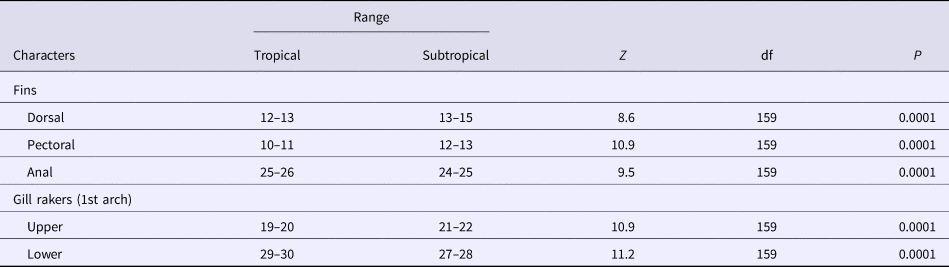
Significant differences were found between the two regions for the number of soft rays of the pectoral, dorsal and the anal fins. The number of soft rays of the pectoral fin differed significantly (Mann–Whitney, Z = 10.9, P < 0.0001) among the two regions, ranging from 10–11 (mean = 10.40, SD = 0.49) in the specimens of the Tropical region (Guandu River and Sepetiba Bay) and from 12–13 (mean = 12.5, SD = 0.36) in those of the Subtropical region (Itiberê River and Paranaguá Bay) (Table 2).
Table 2. Comparisons of the meristic characters of A. januaria between the two regions using the Mann-Whitney U test (Z statistic)
df, degrees of freedom.
The number of dorsal fin rays differed significantly between the two regions (Z = 8.6, P < 0.0001), ranging from 12–13 (mean = 12.89, SD = 0.32) in the specimens of the Tropical region and from 13–15 (mean = 13.96, SD = 0.65) in those from the Subtropical region (Table 2). The number of rays of the anal fin overlapped between the two regions, although significant differences (Z = 9.5, P < 0.001) were found, ranging from 25–26 (mean = 25.53, SD = 0.50) in the specimens of the Tropical region, and from 24–25 (mean = 24.27, SD = 0.45) in those from the Subtropical region (Table 2).
The number of rakers of the first upper branchial arch differed significantly (Z = 10.9, P < 0.001) between the two areas ranging from 19–20 (mean = 19.51, SD = 0.50) in the specimens from the Tropical region, and from 21–22 (mean = 21.40, SD = 0.49) in those from the Subtropical region (Table 2). The number of rakers of the first lower branchial arch differed significantly between the two areas (Z = 11.2, P < 0.0001) ranging from 29–30 (mean = 29.43, SD = 0.49) in the specimens from the Tropical region and from 27–28 (mean = 27.50, SD = 0.50) in those from the Subtropical region (Table 2).
Multivariate analyses: principal components analysis (PCA)
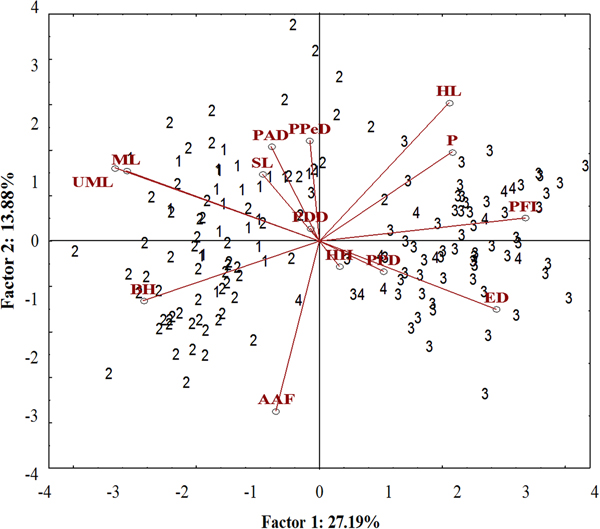
A well-differentiated pattern between specimens from the Tropical (Sepetiba Bay and Guandu River) and those from Subtropical (Paranaguá Bay and Itiberê River) regions was detected in the ordinate diagram of the two first axes from PCA analysis. Specimens from the Tropical and Subtropical regions were separated along the first axis (27.19% of the explained variance). There was overlap between the specimens of the Guandu River and Sepetiba Bay, and between specimens from the Itiberê River with specimens from Paranaguá Bay (Figure 5).
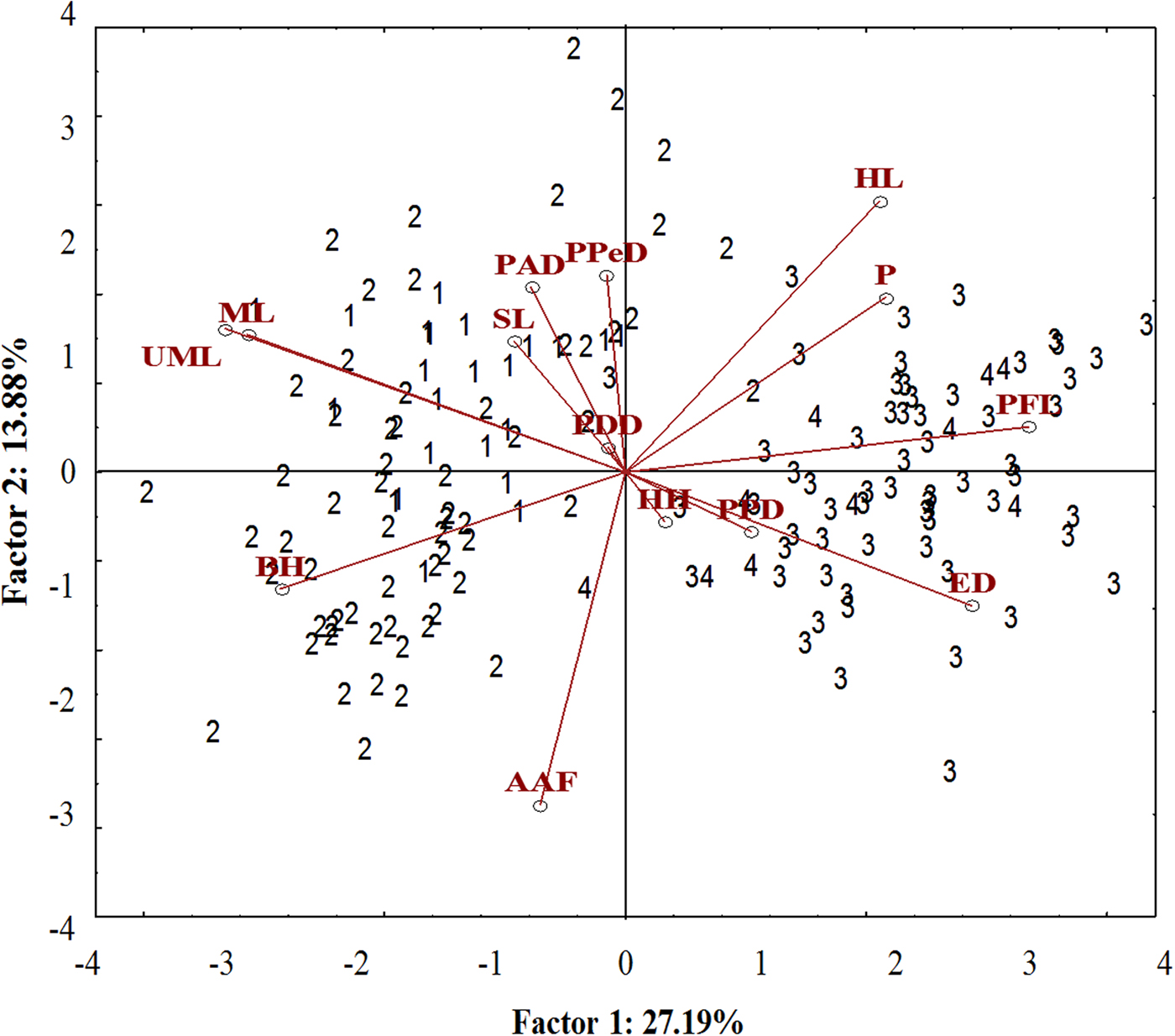
Fig. 5. Scatterplot from the first two axes (scores) from principal component analysis on morphometric data of A. januaria from the four studied areas: 1, Guandu River; 2, Sepetiba Bay; 3, Itiberê River; and 4, Paranaguá Bay. SL, Standard length; HL, Head length; ED, Eye diameter; UML, Upper maxilla length; PFL, Pectoral fin length; ML, Mouth length; PPD, Pre-pectoral distance; PPeD, Pre-pelvic distance; PDD, Pre-dorsal distance; PAD, Pre-anal distance; AAF, Anus-anal fin distance; BH, Body height; HH, Head height; P, Peduncle.
The pectoral fin length, head length, eye diameter and caudal peduncle height had a positive correlation with axis 1, whereas mouth length, upper maxillary length and body height had a negative correlation. A significant positive correlation with axis 2 was detected for the head length and negative for anus-anal fin distance. Specimens from the Tropical region had larger mouth length, maxillary length and body height and smaller pectoral fin length and eye diameter compared with those from the Subtropical region. Although the head length correlated inversely with the anus-anal fin distance, these characters did not differentiate specimens from the two regions (Figure 5). The upper maxillary length and the mouth length had a strong positive correlation with each other and a negative correlation with eye diameter and these characters differentiate specimens from the two regions (Figure 5).
Discriminant analysis
The upper maxillary length and mouth lengths had a positive correlation with the first discriminant axis, whereas the eye diameter and pectoral fin length had a negative correlation (Table 3). Moreover, the head height and body height had a positive correlation with the second discriminant axis, whereas the pre-anal fin and the pre-pectoral fin distances had a negative correlation (Table 3).
Table 3. Canonical discriminant axes for morphometric variables of A. januaria
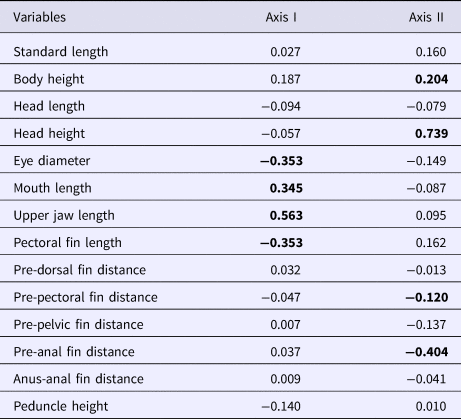
Significant (P < 0.05) correlation in bold.
Per cent of specimens that were correctly classified within their original groups ranged from 60.0–98.4% (Table 4). The highest proportion of correct classification was found for the specimens from the Itiberê River (98.4%) and Sepetiba Bay (95.4%), whereas the specimens from the Guandu River (87%) and Paranaguá Bay (60%) had comparatively lower proportions of correct classification according to the discriminant analysis. On average, 93.2% of the groups were correctly classified (Table 4).
Table 4. Number (above) and per cent (below) of specimens of A. januaria classified according to discriminant analysis on morphometric characters of individuals in the four different areas

Discussion
A different morphometric pattern between specimens of A. januaria from the Tropical (Sepetiba Bay and Guandu River) and Subtropical regions (Paranaguá Bay and Itiberê River) in the Brazilian coast was found. This suggests that morphological divergence occurs, an indication of two separated stocks/populations. Specimens from the Tropical region had comparatively larger body height, mouth length and maxillary length and smaller values for the other characters (e.g. eye diameter, head height, head length and pectoral fin length). These differences indicate that the body structures of this species are not homogeneous in this part of the Brazilian coast, and two distinct groups were depicted for these areas. These findings corroborate the hypothesis described by Cervigón (Reference Cervigón1969) that observed meristic variations in A. januaria specimens from the south-east Brazilian region (latitude 23–24°S) and the North-east (8°S) and proposed that the specimens between these two localities belong to two distinct populations. In general, fish species have great morphological variation both within and between populations when compared with other vertebrates and are more susceptible to morphological divergence induced by the environment in which they live (Wimberger, Reference Wimberger1992; Kelley et al., Reference Kelley, Davies, Collin and Grierson2017). This pattern of variation found for the species may be an indicator of reproductive isolation between individuals from both localities, which could possibly be reinforced by genetic analysis. The Sepetiba Bay (latitude ~23°S) is located in the southern limit of the tropical zone, where tropical conditions are dominant. Moving towards the south, the tropical climate is replaced by a more stable transitional climate, reaching warm temperate conditions in the southern coast of Brazil (Nobre & Shukla, Reference Nobre and Shukla1996).
Morphological divergence has been reported for other members of the Engraulidae family elsewhere. Turan et al. (Reference Turan, Ergüden, Gürlek, Bafiusta and Turan2004) found four groups of E. encrasicolus based on morphological differences in the Black, Aegean and Mediterranean Seas and attributed these dissimilarities to different life histories of each group that belong to different spawning groups and were submitted to different environmental pressures. Lecomte et al. (Reference Lecomte, Grant, Dodson, Rodríguez-Sánchez and Bowen2000) found that morphometric and meristic differences in Engraulis mordax Girard, 1854 may be strongly linked to an ontogenetic response to local environmental conditions. Although E. mordax has a high potential for gene flow because of its high dispersion, many studies have advocated the isolation of populations along the North American coast (e.g. Vrooman et al., Reference Vrooman, Paloma and Zweifel1981; Uribe-Alcocer et al., Reference Uribe-Alcocer, Valdes Morales, Diaz Jaimes, Hornelas Orozco and Arenas1996). These results are corroborated by the findings of Spanikis et al. (Reference Spanikis, Tsimenides and Zouros1989) and Bembo et al. (Reference Bembo, Carvalho, Cingolani, Arneri, Giannetti and Pitcher1996) that reported genetic and morphometric differences in E. encrasicolus between the Aegean and Ionian Seas and between the Aegean and Tyrrhenian Seas.
Thomas et al. (Reference Thomas, Willette, Carpenter and Santos2014) identified genetic and morphological evidence that suggests diversity within Sardinella gibbosa (Bleeker, 1849) collected from eight locations across the Philippine archipelago. Braga (Reference Braga1987) found differences in the relative growth of Sardinella brasiliensis (Steindachner, 1879), a migratory species along the inner platform of south-eastern/southern Brazil, between the coast of Rio de Janeiro (23°S) and Santa Catarina (29°S). They found that differences in environmental conditions, especially the upwelling area at the north coast of Rio de Janeiro State increases feeding availability of phyto- and zooplankton in the area (Gonzalez-Rodriguez et al., Reference Gonzalez-Rodriguez, Valentin and Andre1992) favouring high abundances of this species. Specimens from Rio de Janeiro had higher body height and head length and higher growth rates compared with specimens from Santa Catarina. In addition, the greater pectoral fin length for specimens from the Subtropical region and greater body height for specimens from the Tropical region may be related to differences in abiotic conditions, a constraint that leads to population morphological divergence (Nosil, Reference Nosil2012; Kelley et al., Reference Kelley, Davies, Collin and Grierson2017).
Several factors may be contributing to morphological differences in A. januaria. Adult and juvenile individuals dwell in estuaries and sandy beaches with low salinities. Adults may use the estuary as a migratory route to lower river reaches to spawn (Silva & Araújo, Reference Silva and Araújo2000) limiting their larval dispersal potential to a smaller distance when compared with marine species that spawn in more open areas. Thus, A. januaria from these two locations belong to different spawning groups, and individuals from these two reproductive groups do not have direct genetic contact since they do not tolerate high salinities (Silva & Araújo, Reference Silva and Araújo2000). In addition, this species has a short longevity which may favour the accumulation of greater genetic variations, as observed between the two studied groups from Tropical and Subtropical regions. Zi-Niu et al. (Reference Zi-Niu, Xiao-Yu, Tian-Hui, Yan-Yan, Zhi-Meng and Xian-Shi2005) found a high level of DNA variation in Japanese anchovy which have spawning behaviour close to beaches and then return to the seas to feed, with a high level of dispersion between the south–south-east regions of the Chinese coast. They attributed these characteristics to a short life cycle when compared with other species with longer lifespans, such as cod. These findings reinforce our hypothesis that the two groups analysed probably come from two different spawning groups.
Meristic characters also showed differences between the two groups, as the number of fin rays tend to be higher in the Subtropical region. Several studies have reported that fishes from lower temperatures tend to have higher numbers of meristic characters compared with those from higher temperatures (Murray & Beacham, Reference Murray and Beacham1989; Sfakianakis et al., Reference Sfakianakis, Leris, Laggis and Kentouri2011). The comparatively lower growth rates in lower temperatures in higher latitudes, with spawning occurring during the summer, favour less energy usage compared with individuals from lower latitudes. This favours greater longevity and the formation of a large number of structures, i.e. meristic characters (Ciechomski & Weiss, Reference Ciechomski and Weiss de Vigo1971; Brett, Reference Brett1979). Mazzetti (Reference Mazzetti1983) compared meristic data of Engraulis anchoita (Hubbs & Marini 1935) from Guanabara Bay (23°S) to those from Argentine waters (Plaza & Boschi, Reference Plaza and Boschi1958), and found that the number of anal and dorsal fin rays from specimens from south-east Brazil (23°S) is always lower than those from Argentine colder waters. The comparatively higher numbers of meristic characters (except the number of anal fin rays) for specimens from the Subtropical region found in the present study corroborates this hypothesis. Paranaguá Bay has lower water temperatures (18–29°C) and salinity (12–34) (Hackradt et al., Reference Hackradt, Félix-Hackradt, Pichler, Spach and Santos2011) compared with Sepetiba Bay that has temperatures from 20–30°C and salinity of 28–33% (Araújo et al., Reference Araújo, Azevedo and Guedes2016). In addition, Paranaguá Bay is characterized by large amounts of fresh and salt water wedges that interact giving rise to great contrasts of salinity during the ebb and flow of the tides. Although meristic characters are mainly used as taxonomic features for interspecific differentiation and are relatively constant in species, they may reflect genetic differences between specific groups (Vidalis et al., Reference Vidalis, Markakis and Tsimenides1997). Thus, it is necessary that population differentiation based on morphological and meristic characters be correlated with genetic studies of the species (Turgeon et al., Reference Turgeon, Reid, Bourret, Pratt, Reist, Muir and Howland2016).
In this study, we found morphological and meristic divergences in A. januaria between the two studied regions. This may be the result of an adaptation to the habitat and the isolation of the specimens in these regions. Further studies using genetic information should be done to confirm our hypothesis of different stocks/populations of A. januaria in these two regions.
Acknowledgements
The authors thank technicians from the Laboratory of Fish Ecology for helping in fieldwork. We also thank the staff from Museu de Zoologia, Universidade de São Paulo, for giving access to fishes from the Subtropical region.
Financial support
This work was funded by FAPERJ – Carlos Chagas Foundation for Supporting Research of the Rio de Janeiro State, CNE Process: E-26/102.997/2011 and by CNPq – Brazilian National Council for Research Development. (Grants for the last author, Process: 304954/2011-0.) This research was conducted under SISBIO Collection of Species Permit number 10707 issued by ICMBio, Brazilian Environmental Agency.