INTRODUCTION
Aspidochirotid holothuroids are a conspicuous component of the macro-benthos of marine environments. As deposit feeders, they process vast quantities of sediment, and thus play an important role in the mineralization and cycling of nutrients in benthic habitats (Uthicke & Klumpp, Reference Uthicke and Klumpp1998; Uthicke, Reference Uthicke1999, Reference Uthicke2001; Purcell et al., Reference Purcell, Conand, Uthicke and Byrne2016; Lee et al., Reference Lee, Ferse, Ford, Wild, Mangubhai, Mangubhai, Lalavanua and Purcell2017). The release of nitrogenous waste by aspidochirotids can increase the productivity of benthic microalgae and seagrass systems, a particularly important functional role in oligotrophic coral reef ecosystems (Uthicke & Klumpp, Reference Uthicke and Klumpp1998; Uthicke, Reference Uthicke2001; Eriksson et al., Reference Eriksson, Torre-Castro, Eklof and Jiddawi2010; Wolkenhauer et al., Reference Wolkenhauer, Uthicke, Burridge, Skewes and Pitcher2010; Purcell et al., Reference Purcell, Conand, Uthicke and Byrne2016; Wolfe & Byrne, Reference Wolfe and Byrne2017a, Reference Wolfe and Byrneb). The digestion and dissolution of carbonate sands by tropical holothuroids can also influence local biogeochemistry by increasing local alkalinity, potentially buffering against ocean acidification and enhancing reef resilience (Hammond, Reference Hammond1981; Schneider et al., Reference Schneider, Silverman, Woolsey, Eriksson, Byrne and Caldeira2011, Reference Schneider, Silverman, Kravitz, Rivlin, Schneider-Mor, Barbosa, Byrne and Caldeira2013; Wolfe et al., Reference Wolfe, Vidal-Ramirez, Dove, Deaker and Byrne2018).
Many holothuroid species are harvested for the lucrative dried seafood trade, with the dried body wall product (bêche-de-mer) highly prized in the Asian market (Purcell et al., Reference Purcell, Mercier, Conand, Hamel, Toral-Granda, Lovatelli and Uthicke2013, Reference Purcell, Conand, Uthicke and Byrne2016; Eriksson & Byrne, Reference Eriksson and Byrne2015; Eriksson & Clarke, Reference Eriksson and Clarke2015). Many commercial species are in a perilous state of conservation with 16 species, largely from tropical regions, recently listed as Threatened with extinction by the International Union for Conservation of Nature (IUCN) (Purcell et al., Reference Purcell, Mercier, Conand, Hamel, Toral-Granda, Lovatelli and Uthicke2013, Reference Purcell, Polidoro, Hamel, Gamboa and Mercier2014; Conand et al., Reference Conand, Polidoro, Mercier, Gamboa, Hamel and Purcell2014). At least 70% of the world's tropical holothuroid fisheries are considered exploited, over-exploited or depleted (Purcell et al., Reference Purcell, Mercier, Conand, Hamel, Toral-Granda, Lovatelli and Uthicke2013), with many species now locally extinct (Hasan, Reference Hasan2005; Anderson et al., Reference Anderson, Flemming, Watson and Lotze2011; Branch et al., Reference Branch, Lobo and Purcell2013; Price et al., Reference Price, Evan, Rowlands and Hawkins2013; Purcell et al., Reference Purcell, Polidoro, Hamel, Gamboa and Mercier2014).
Despite the important ecological roles of commercial holothuroids in coral reef ecosystems and their commercial value, we have a limited understanding of their reproductive cycles and spawning periodicity. These animals are iteroparous and investigation of 11 Indo-Pacific species including some of the most commercially valuable (Holothuria fuscogilva, Actinopyga mauritiana, Thelenota ananas) indicates that they spawn annually during summer (Conand, Reference Conand1981, Reference Conand1993a, Reference Conandb; Conand et al., Reference Conand, Uthicke and Hoareau2002; Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2003). Spawning during winter is also documented including for H. whitmaei (Conand, Reference Conand1981, Reference Conand1993a, Reference Conandb; Ramofafia et al., Reference Ramofafia, Battaglene, Bell and Byrne2000, Reference Ramofafia, Byrne and Battaglene2001; Shiell & Uthicke, Reference Shiell and Uthicke2006; Asha & Muthiah, Reference Asha and Muthiah2008). While less common, biannual spawning has been observed for H. atra in New Caledonia (Conand, Reference Conand1993a, Reference Conandb). The timing of spawning can differ among geographically separated conspecific holothurian populations (Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2003). Holothuria scabra spawns year round in equatorial regions (Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2003), but spawns annually in higher latitude regions (Conand, Reference Conand1993a, Reference Conandb). These differences in spawning within a species are largely restricted to populations separated by latitudinal (north-south) oriented differences (Shiell & Uthicke, Reference Shiell and Uthicke2006). Due to the threatened status of commercial sea cucumbers, globally, we need a better understanding of their reproductive biology as a key fishery biology trait.
The curryfish, Stichopus herrmanni, is an abundant Indo-Pacific sea cucumber distributed from East Africa to Australia and Indonesia, which inhabits sandy lagoons, seagrass and coral reef habitats between depths of 0–30 m (Conand, Reference Conand1993a, Reference Conandb; Eriksson et al., Reference Eriksson, Thorne and Byrne2013; Wolfe & Byrne, Reference Wolfe and Byrne2017a, Reference Wolfe and Byrneb). Stichopus herrmanni, a mid- to low-value bêche-de-mer species, has become a major harvest target as populations of higher value species continue to decline (Purcell et al., Reference Purcell, Mercier, Conand, Hamel, Toral-Granda, Lovatelli and Uthicke2013, Reference Purcell, Polidoro, Hamel, Gamboa and Mercier2014; Eriksson & Byrne, Reference Eriksson and Byrne2015). Between 2007 and 2011, curryfish catches along the Great Barrier Reef (GBR) Marine Park, increased at an average annual rate of 200% (Eriksson & Byrne, Reference Eriksson and Byrne2015). Overall fishing pressure has resulted in a 60–90% population decline over half of the species' global range (Conand et al., Reference Conand, Polidoro, Mercier, Gamboa, Hamel and Purcell2014). Stichopus herrmanni is listed as Vulnerable to extinction by the IUCN (Purcell et al., Reference Purcell, Polidoro, Hamel, Gamboa and Mercier2014; Eriksson & Byrne, Reference Eriksson and Byrne2015).
We investigated the reproductive biology of S. herrmanni at One Tree Island (OTI), southern GBR. Our current understanding of the reproduction of S. herrmanni is based on data obtained from a population in New Caledonia, where spawning over 5 years occurred in summer (Conand, Reference Conand1993a, Reference Conandb). Thus far, the reproductive cycle of only two bêche-de-mer species has been determined for populations on the GBR; H. whitmaei spawns in winter and H. atra spawns in summer (Shiell & Uthicke, Reference Shiell and Uthicke2006; Lee et al., Reference Lee, Byrne and Uthicke2008; Thorne et al., Reference Thorne, Eriksson and Byrne2012). We determined the timing of gonad development and gamete maturation for S. herrmanni using gonad histology and the gonad index method. We also compiled observations of spawning by S. herrmanni in situ at OTI and elsewhere along the GBR and the Indo-Pacific. We hypothesized that reproduction of this species at OTI would be similar to that observed in New Caledonia (Conand, Reference Conand1993a, Reference Conandb), as OTI and New Caledonia exist at similar latitudes. Our goal was to identify the spawning period of S. herrmanni at OTI and where possible, elsewhere on the GBR, providing important new information for the curryfish fishery to facilitate development of sustainable fisheries practices for this key bêche-de-mer species.
MATERIALS AND METHODS
Stichopus herrmanni (120–600 mm length) were collected from the lagoon (2–4 m depth) at One Tree Island (OTI) (23°30′S 152°05′E), southern GBR. Due to permit restrictions (GBR Marine Park Authority Permit G13/36027.1.) on the total number of sea cucumber species allowed to be collected annually we used data from collections over many years (Jan 2009, 2016, Feb 2011, 2013, Apr 2012, May 2009, Jul 2009, Aug 2015, Sep 2009, Oct 2013, Nov 2008, Dec 2009). We combined data per collection month across years. To determine the gonad index, S. herrmanni were dissected and the coelomic fluid drained. The gonad index was calculated as the weight % of the gonads relative to the combined drained body wall and viscera weights. Averages were taken per month. The seasonal temperature cycle of the lagoon was determined from a long-term monitoring programme (http://data.aims.gov.au/aiemsrtds/station.html?station=131). Average daily temperatures in OTI lagoon were combined to calculate monthly averages between 2008 and 2017 (N = 66–99 month−1).
A portion of the gonad was fixed in Bouin's fluid and processed for routine wax histology. The gonad sections (7 µm thick) were stained in haematoxylin and eosin, and gonad histological condition was determined by microscopic examination. The gametogenic state of the gonads were scored in four stages: mature, partly spawned, post-spawned/spent and recovering, as in previous studies of sea cucumber gonad histology (Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2001, Reference Ramofafia, Byrne and Battaglene2003). Digital images of gonad sections were captured using an Olympus DP70 digital camera mounted onto an Olympus BX50 microscope. Maximum egg size in mature ovaries and the thickness of the gonad wall of testes and ovaries were determined using Image-J (NIH, Bethesda, MD, USA).
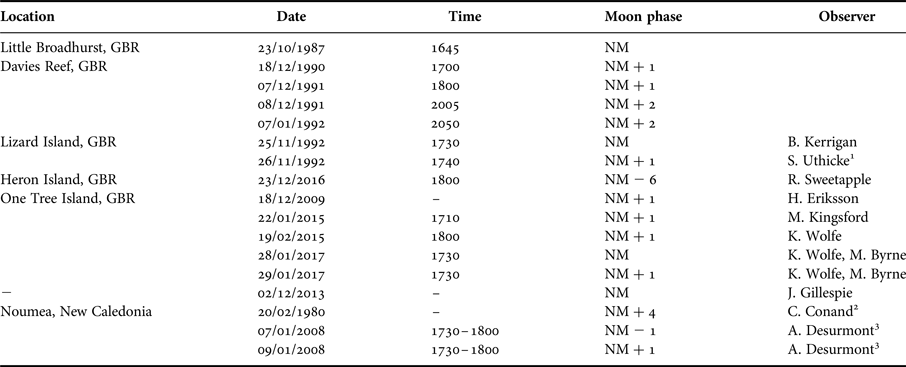
Observations of spawning of S. herrmanni with information of the date and time of gamete release were assimilated from personal and published observations collected at OTI, elsewhere along the GBR and at other locations in the Pacific (see Table 1). Data were assessed with respect to the lunar cycle. Based on the pattern that emerged (see Results), we undertook a targeted survey for spawning behaviour at OTI around the new moon in January 2017. On three days (28–30 January, 17:30–18:30 h) coinciding with the new moon (NM), NM + 1 day and NM + 2 days, the number of spawning individuals were counted in the first 18–20 individuals randomly encountered along two transects (10–20 m) along the reef edge at Shark Alley (0.5–3 m depth), OTI, on snorkel.
Table 1. Observations of Stichopus herrmanni spawning on the Great Barrier Reef between 1987 and 2016, with indication of lunar cycle (NM = new moon). Data taken from: 1. Uthicke (Reference Uthicke1994); 2. Conand (Reference Conand1989); 3. Desurmont (Reference Desurmont2008).
To determine whether the gonad index differed among months, these data were compared by one-way ANOVA, with month as the fixed factor. Percentage data were arcsine transformed before analysis using JMP 501 (Cary, NC, USA). As required for ANOVA, homogeneity of variance and normality was checked and confirmed for all data series (Quinn & Keough, Reference Quinn and Keough2003). Post-hoc Tukey's HSD tests were used to determine where significant differences lay.
RESULTS
Ovary histology
Mature ovaries Stichopus herrmanni had late stage oocytes (mean diameter: 94.3 ± 0.9 µm; N = 420) filling the lumen, each located in an individual follicle (Figure 1A, B). During the mature stage the ovary wall was at its minimal thickness (14.3 ± 1.5 µm; N = 120). Partly spawned ovaries were marked by loosely packed unspawned oocytes and the presence of aggregations of phagocytes in the lumen (Figure 1C, D). Overlapping generations of oocytes were present in mature and partly spawned ovaries with a renewal of gametogenesis evident along the germinal epithelium (Figure 1C, D). Fully mature and partly spawned gonads were observed in late spring and summer (Nov–Feb). The largest oocytes had a mean diameter of 101.1 ± 2.8 µm (N = 120) (Figure 1B).
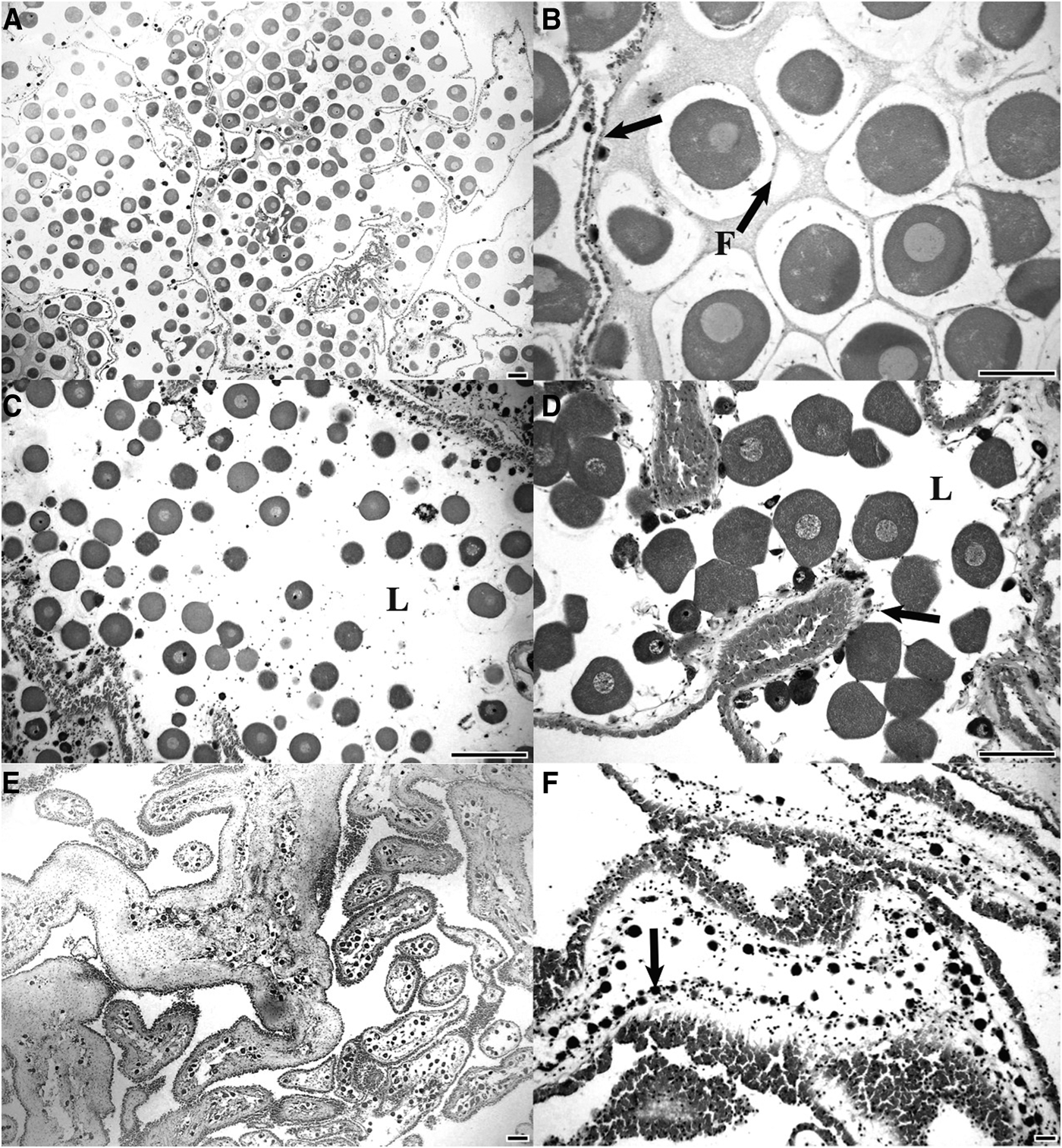
Fig. 1. Ovary histology of Stichopus herrmanni. (A, B) Mature ovary with the lumen filled with late stage oocytes within individual follicles (F). Note the thin gonad wall (arrow); (C, D) partly spawned ovary with oocytes scattered in the lumen (L) and early developing oocytes along the germinal epithelium (arrow); (E) spent ovaries with a few remaining oocytes and a thick wall; (F) recovering ovary with a new cohort of developing oocytes along the germinal epithelium (arrows) (scale bars = 200 µm).
Spent ovaries of S. herrmanni were largely empty and had a thick gonad wall (27.7 ± 2.0 µm; N = 120) (Figure 1E). Variable numbers of relict oocytes and phagocytes were present. It appears that the unspawned eggs were broken down and reabsorbed. Recovery stage ovaries had basophilic previtellogenic oocytes developing along the germinal epithelium (Figure 1F). As the oocytes developed they became increasingly eosinophilic, indicating vitellogenesis.
Testis histology
Mature testes were packed with basophilic spermatozoa (Figure 2A, B). Infolds of the germinal epithelium were absent and the wall of the testis was at its thinnest (7.0 ± 0.8 µm; N = 120). Partly spawned testes had fewer sperm in the lumen and an increase in the thickness of the gonad wall (Figure 2C, D). There was evidence of renewed spermatogenesis in partly spawned testes with developing sperm present along the re-appeared folds of the germinal epithelium. Spent testes were devoid of contents, with the exception of a few unspawned spermatozoa (Figure 2E) and the gonad wall was thick (24.1 ± 1.7 µm; N = 120) and had a wrinkled appearance. In recovery stage testes, developing sperm lined the germinal epithelium. The layer of developing sperm increased as the testis developed with invaginations of the germinal epithelium protruding towards the centre of the tubule, increasing the surface area of the germinal epithelium (Figure 2F).
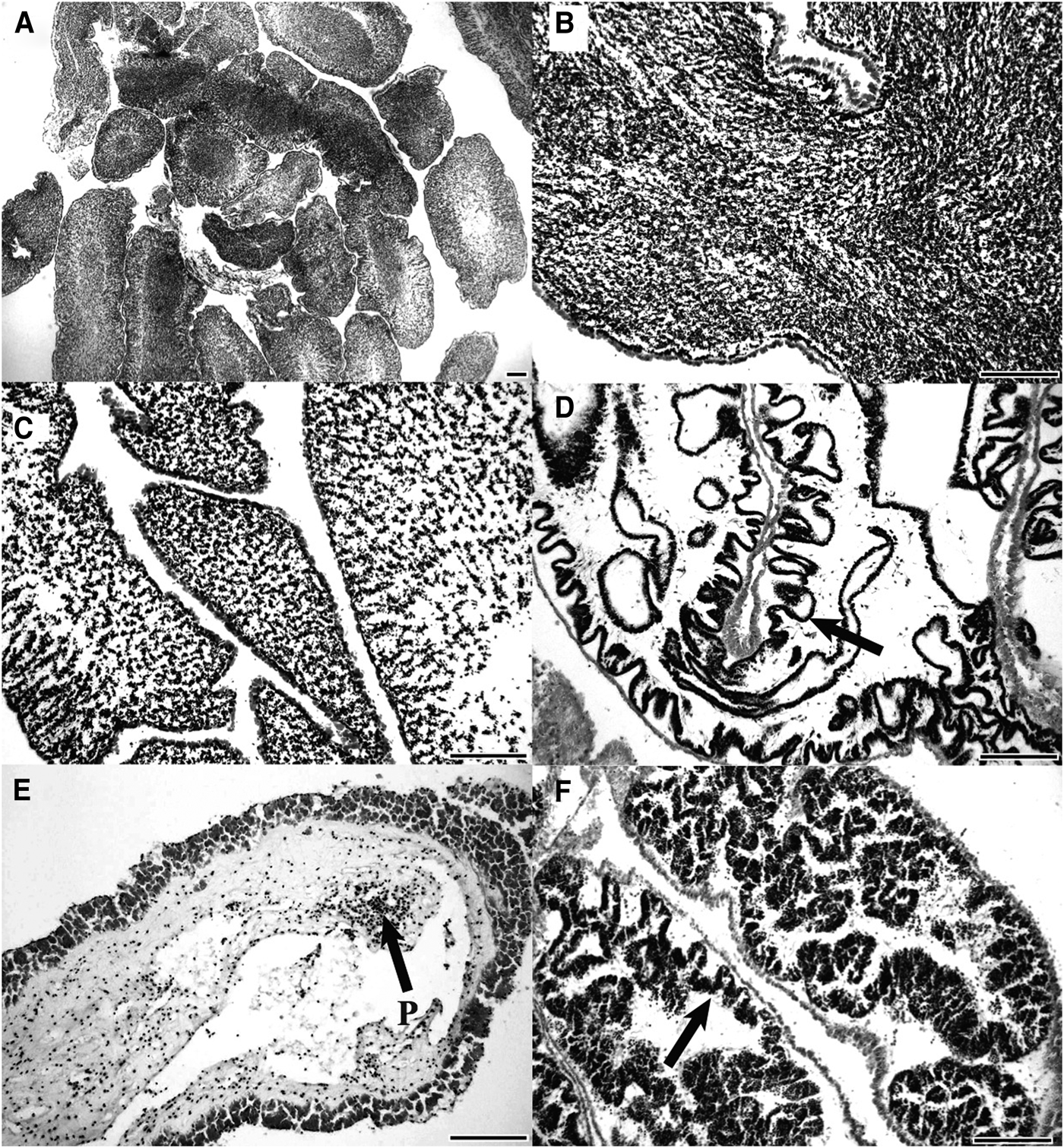
Fig. 2. Testis histology of Stichopus herrmanni. (A, B) Mature testis with the lumen filled with sperm; (C) partly spawned testis with sperm scattered in the lumen; (D, E) spent testis with an empty lumen. The folds of the germinal epithelium are evident (arrows) as are aggregations of phagocytes (P); (F) recovering testis with a new cohort of developing sperm along the folds of the germinal epithelium (scale bars = 200 µm).
Seasonal trends in histology and the gonad index
Gametogenesis of S. herrmanni showed a seasonal pattern (Figure 3). In both sexes, mature gametes were observed from late-spring (Nov, 25%) through summer (Dec, 80%; Jan, 5.6%; Feb, 64.7%). In summer, most S. herrmanni were mature (Figure 3). Partly spawned individuals were also present in summer, especially in January (77.8%). Almost all S. herrmanni observed in autumn (April–May) and winter (July–Aug) had spent gonads. Recovery stage gonads were evident from August (55%) through to November (75%).

Fig. 3. Histological condition of the gonads of Stichopus herrmanni (sample size in parentheses) across the seasons.
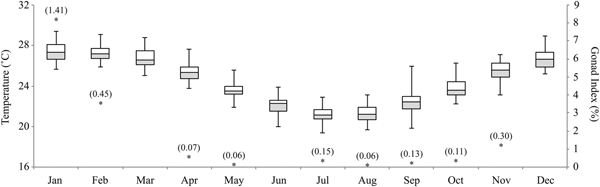
OTI lagoon has a distinct seasonal temperature cycle, with daily averages ranging from 25.2–29.4°C in the summer months (mean: Dec = 26.7°C, Jan = 27.3°C, Feb = 27.2°C), to 19.4–23.9°C in the winter months (mean: Jun = 22.3°C, Jul = 21.2°C, Aug = 21.2°C) (Figure 4). The annual mean daily temperature was 24.4°C (± 0.7), with an average range of 19.4–29.4°C across the year. The gonad index of S. herrmanni differed between months (F 8,59 = 10.16, P < 0.0001), in parallel with water temperature (Figure 4). Tukey's HSD tests revealed that the average gonad index was highest in January (8.26 ± 1.4%) and February (3.65 ± 0.5%) (Figure 4). Some S. herrmanni lacked identifiable gonads in the winter.

Fig. 4. Annual temperatures at One Tree Island lagoon (2008–2017) and gonad index for Stichopus herrmanni (sample size in parentheses).
Spawning observations
Records from in situ observations (N = 13) show that Stichopus herrmanni spawns from late spring (Nov) through summer (Dec–Feb) near sunset (1730–1830) (Figure 5), typically around the new moon at several sites along the GBR from the southern (One Tree Island, Heron Island), central (Little Broadhurst Reef, Davies Reef), northern (Lizard Island) GBR, and also in New Caledonia (Table 1). One spawning observation was made ~1 week before a new moon event in December 2016 (Table 1). As is typical of aspidochirotids, S. herrmanni adopts an erect posture during spawning (Figure 6A).
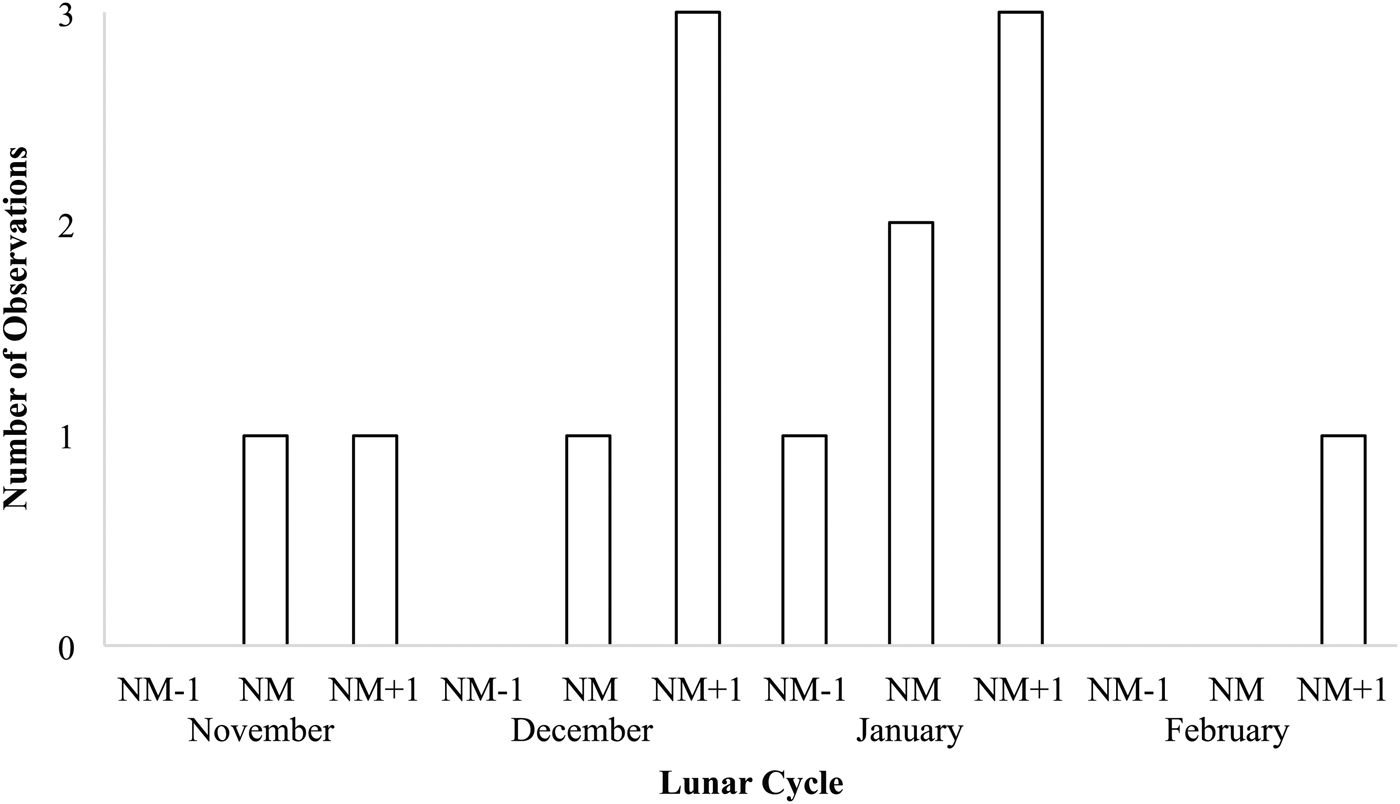
Fig. 5. Number of observations of spawning of Stichopus herrmanni on the Great Barrier Reef and New Caledonia with respect to lunar cycle (NM = new moon).

Fig. 6. Broadcast spawning behaviour of Stichopus herrmanni on One Tree Island, showing the (A) erect posture typical of spawning holothuroids and (B) sperm release. Spawning individuals attract fishes (arrow) to the released gametes.
Observations of spawning by S. herrmanni were most frequently documented in January with additional observations in November, December and February (Figure 5). Overall, most spawning observations were recorded on the day following the new moon, as well as on the new moon and a day before the new moon. Of the months where spawning was recorded, February had the lowest number of spawning events, with only one event recorded on the day after a new moon. There was a single outlier observation, a report of spawning in late October (Table 1).
In the surveys conducted at OTI on 28–29 January 2017 (new moon and one day following) (Table 1), spawning was observed late afternoon of the NM (5/20 spawning) and NM + 1 (16/21 spawning). No spawning was observed on NM + 2 (Table 1). Males spawned before females. As sunset approached individuals were still in the process of moving to elevated positions (Figure 6A). Small fishes attacked the anterior end of spawning individuals and appeared to be consuming the released gametes (Figure 6B).
DISCUSSION
Stichopus herrmanni has an annual reproductive cycle at OTI, spawning from late spring through summer. This pattern, based on evidence from gonad index, gonad histology and spawning observations, is similar to that reported for this species in New Caledonia in a 5-year study (Conand, Reference Conand1993a, Reference Conandb), at a similar latitude to the population investigated here. Summer spawning is reported for several tropical aspidochirotids, including other stichopodids (e.g. Thelonata ananas) and holothurids (e.g. Holothuria atra) (Conand, Reference Conand1981, Reference Conand1993a, Reference Conandb; Conand et al., Reference Conand, Uthicke and Hoareau2002; Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2003). Although our data for elsewhere on the GBR is limited, the spawning observations suggest that S. herrmanni spawns during the summer in this region.
Stichopus herrmanni at OTI has an annual gametogenic cycle. Oocytes developed from late winter to summer with the fully grown oocytes evident from November, as also reported in New Caledonia (Conand, Reference Conand1993a, Reference Conandb). The histological condition of the gonads indicated that spawning in S. herrmanni is partial with fully mature and partly spawned gonad tubules present throughout summer. Spawning observations also indicate that S. herrmanni has episodic gamete release (i.e. multiple spawning) through late-spring and summer. By April, the gonads were post-spawned and the remaining gametes appear to be reabsorbed. Resorption of unspawned gametes is a common feature of aspidochirotid and echinoderm gonads and is suggested to be associated with exogenous cues and/or an underlying endogenous rhythm (Eckelbarger & Young, Reference Eckelbarger and Young1992; Morgan, Reference Morgan2000; Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2003). After the spawning period, the gonads enter a spent/resting phase with little gametogenic activity for 6 months over winter (March/April to Sept/Oct) as also reported for S. herrmanni in New Caledonia (Conand, Reference Conand1993a, Reference Conandb).
Temperature, salinity, photoperiod and food availability have been suggested to regulate the process of gametogenesis in sea cucumbers (Cameron & Fankboner, Reference Cameron and Fankboner1986; Conand, Reference Conand1993a, Reference Conandb; Morgan, Reference Morgan2000; Hamel et al., Reference Hamel, Conand, Pawson and Mercier2001; Conand et al., Reference Conand, Uthicke and Hoareau2002; Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2003; Shiell & Uthicke, Reference Shiell and Uthicke2006). The gonads of S. herrmanni had minimum development in winter and were mature in summer, indicating gametogenesis is controlled by temperature and day length. In both temperate and tropical species, the early gametogenic phase appears to coincide with the cooler months and when days are shorter than nights, as suggested for Actinopyga mauritiana, H. scabra, S. californicus, Thelenota ananas (Cameron & Fankboner, Reference Cameron and Fankboner1986; Conand, Reference Conand1993a, Reference Conandb; Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2001). A resting phase in the winter, when the gonads are at their minimal size (or absent), is a feature described for temperate stichopodids, and is suggested to represent an aestivation-like cessation of activity due to cold conditions at high latitudes (Cameron & Fankboner, Reference Cameron and Fankboner1986). This phenomenon is also observed for tropical species, indicating that a quiescent gonad phase may be common among aspidochirotids (Morgan, Reference Morgan2000; Hamel et al., Reference Hamel, Conand, Pawson and Mercier2001; Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2003).
At OTI, S. herrmanni experiences distinct seasonal temperature fluctuations (~10°C), and the cold season might initiate an aestivation-like response in reproductive activity. However, S. herrmanni is abundant in equatorial locations where annual temperature flux is minimal. In these regions with minimal seasonal cues, continuous reproduction is predicted for echinoderms (Giese & Pearse, Reference Giese and Pearse1974) as occurs in equatorial populations of H. scabra (Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2003). An assessment of the reproductive cycle of low latitude populations of S. herrmanni would provide insight into whether a gonadal resting phase is species-specific or varies with latitude (i.e. temperature-driven). In addition to the seasonal temperature cycle, day length and lunar cycle, which are fairly predictable among years, it would also be important to consider the influence of other environmental variables such as food availability and cyclones on reproduction of S. herrmanni.
The onset of gonad growth occurred in late-winter (August), correlating with increasing day length. There was an overlap in spent and recovering stages of gametogenesis in the gonads of S. herrmanni at OTI from August to October. Gonad maturation is reached by November at OTI and New Caledonia (Conand, Reference Conand1993a, Reference Conandb), as temperature and day length increase. Summer spawning indicates that the small individuals observed in autumn (four months later) on nearby reefs are likely to be new recruits (Wolfe & Byrne, Reference Wolfe and Byrne2017b).
While gonad development and maturation appear to be entrained by a seasonal cycle, coinciding with the warmest temperatures and longest days, spawning and the behavioural change to move to elevated places such as coral bommies appears to be cued by the lunar cycle. This behavioural change brings individuals closer together, an aggregation-like response also observed in H. scabra (Morgan, Reference Morgan2000; Hamel et al., Reference Hamel, Conand, Pawson and Mercier2001). At OTI, S. herrmanni began moving up coral bommies before dusk and spawning started soon after, between 1645 and 2050 h. It is likely that a more aggregated distribution and spawning continues into the night, as for S. chloronotus on the GBR (Babcock et al., Reference Babcock, Mundy, Keesing and Oliver1992). Observations in the Solomon Islands indicate that H. scabra are normally scattered but progressively form pairs, trios and larger groups, peaking a few days before the new moon. Pheromones released by males have been shown to induce aggregation and spawning in H. arguinensis and sympatric holothuroids (Marquet et al., Reference Marquet, Hubbard, da Silva, Afonso and Canario2018). Aggregative spawning is suggested to be an evolved strategy to increase fertilization rates and reduce predation of gametes through predator satiation (Babcock et al., Reference Babcock, Mundy, Keesing and Oliver1992). As for S. herrmanni, males spawn prior to females in other aspidochirotids (Babcock et al., Reference Babcock, Mundy, Keesing and Oliver1992; Morgan, Reference Morgan2000; Hamel et al., Reference Hamel, Conand, Pawson and Mercier2001; Marquet et al., Reference Marquet, Hubbard, da Silva, Afonso and Canario2018). Males release sperm over long periods in contrast to the short bursts of egg release by females (Babcock et al., Reference Babcock, Mundy, Keesing and Oliver1992).
The timing of spawning can differ among geographically separated conspecific holothurian populations (Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2003). The tropical sea cucumber H. scabra spawns all year round in the Solomon Islands near the equator and annually in higher latitude regions, a disparity reflecting latitudinal (north-south) differences (see review, Ramofafia et al., Reference Ramofafia, Byrne and Battaglene2003). For H. whitmaei, spawning occurs in winter for western and eastern Australian populations at the same latitude, in the Indian and Pacific Oceans, respectively (Shiell & Uthicke, Reference Shiell and Uthicke2006). As S. herrmanni is such a widely distributed species in the Indo-Pacific, it remains to be determined if its reproductive cycle and spawning periodicity differs across oceans and latitude.
Successful reproduction of marine invertebrates requires individuals to be in close proximity (Pakoa et al., Reference Pakoa, Masu, Teri, Lequata, Tua, Fisk and Bertram2014). Low population densities effectively reduce the chances of the successful fertilization, leading to reproductive failure – the Allee effect (Allee, Reference Allee1938). Unlike several tropical holothuroids that can reproduce asexually (e.g. H. atra, Lee et al., Reference Lee, Byrne and Uthicke2008), S. herrmanni only reproduces by sexual means. Weakened mating capacity of stocks due to overfishing leads to population declines and local extinctions with minimal population recovery, as observed for several commercial species (Uthicke et al., Reference Uthicke, Welch and Benzie2004; Hasan, Reference Hasan2005; Purcell, Reference Purcell2010; Friedman et al., Reference Friedman, Eriksson, Tardy and Pakoa2011). Although the population density of S. herrmanni is particularly high on OTI (Eriksson et al., Reference Eriksson, Thorne and Byrne2013; Wolfe & Byrne, Reference Wolfe and Byrne2017a), which has been an unfished reef for decades, populations on reefs open to fishing are vulnerable to exploitation. There are no pre- or post-harvest data for any of the fished areas on the GBR, so the impact of removal of mature adults on reproductive success and the persistence of populations is not known. This is an urgent knowledge gap to address considering the Vulnerable status of S. herrmanni (Eriksson & Byrne, Reference Eriksson and Byrne2015).
Early research on the primary high-value target bêche-de-mer species on the GBR, H. whitmaei, prompted the closure of its fishery over a decade ago (Roelofs, Reference Roelofs2004; Uthicke et al., Reference Uthicke, Welch and Benzie2004). As populations of high-value species decline, low- to mid-value species are targeted leading to a sequential pattern of depletion across the Indo-Pacific (Purcell et al., Reference Purcell, Polidoro, Hamel, Gamboa and Mercier2014; Eriksson & Byrne, Reference Eriksson and Byrne2015; Eriksson & Clarke, Reference Eriksson and Clarke2015). As a result, the mid-value curryfish, S. herrmanni, is now a major target species on the GBR (Eriksson & Byrne, Reference Eriksson and Byrne2015). It is likely that the commercial exploitation of S. herrmanni will mimic the patterns documented for previous target species if appropriate management strategies are not implemented (Eriksson & Byrne, Reference Eriksson and Byrne2015; Eriksson et al., Reference Eriksson, Conand, Lovatelli, Muthiga and Purcell2015). Based on our data, spawning closures could be considered. At present there are no constraints on fishing throughout the year for S. herrmanni. Importantly, this species exhibits an ontogenetic migration within their recruitment reef (Eriksson et al., Reference Eriksson, Thorne and Byrne2013; Wolfe & Byrne, Reference Wolfe and Byrne2017b). This connectivity between juvenile recruitment and adult habitats highlights the vulnerability of many marine species to overfishing (Gillanders et al., Reference Gillanders, Able, Brown, Eggleston and Sheridan2003; Grüss et al., Reference Grüss, Kaplan, Guénette, Roberts and Botsford2011). However, there are no data available on S. herrmanni, and many other target commercial species, on fished reefs along the GBR.
Current approaches of bêche-de-mer fisheries are extremely exploitive and are not viable for holothuroid resources (Conand et al., Reference Conand, Polidoro, Mercier, Gamboa, Hamel and Purcell2014; Purcell et al., Reference Purcell, Samyn and Conand2012, Reference Purcell, Mercier, Conand, Hamel, Toral-Granda, Lovatelli and Uthicke2013, Reference Purcell, Polidoro, Hamel, Gamboa and Mercier2014; Eriksson & Byrne, Reference Eriksson and Byrne2015), prompting the suggestion that a paradigm shift in fisheries management is needed. Fisheries-independent information is necessary to understand the true status of the commercial fishery on the GBR, and elsewhere. This is particularly important with regard to the aggregative behaviour exhibited during spawning and the clear implications for negative Allee effects following unsustainable fishing practices.
ACKNOWLEDGEMENTS
We thank many colleagues for assistance with data collection, particularly Dr Brendan Kelaher and Dr Mel Coleman and to colleagues who provided precise information on spawning over years including Dr Mike Kingsford and Dr Hampus Eriksson. Thanks to the staff of One Tree Island Research Station, a facility of the University of Sydney. This project operated under the GBRMPA permit no. G13/36027.1.
FINANCIAL SUPPORT
This research was supported by grants from the Mohamed bin Zayed Species Conservation Fund, the Great Barrier Reef Foundation, the Great Barrier Reef Marine Park Authority, the Paddy Pallin Foundation, the Holsworth Wildlife Research Endowment and a PhD Scholarship from the University of Sydney.