Introduction
Ctenophora are a small and well-defined group of planktonic and benthic predators, they can be found exclusively in most marine habitats, from polar to tropical, inshore to offshore and from near the surface to the deep sea and benthic environments (Mianzan et al., Reference Mianzan, Dawson, Mills and Gordon2009; Mills, Reference Mills2010). They are sometimes called ‘comb jellies’ because they have a jelly-like appearance and distinctive comb rows or cilia plates (ctenes) used for locomotion (Tamm, Reference Tamm2014). Comb jellies, except those in the family Beroidae (Carré and Carré, Reference Carré and Carré1989), have two opposed and fast ductile tentacles for catching and delivering prey to their digestive tract (von Byern et al., Reference von Byern, Mills, Flammang, von Byern and Grunwald2010). Nevertheless, some of this and other morphological characteristics are absent in benthic ctenophores; most have lost the ctene rows as adults (as an adaptation to bottom condition) and, thus, the ability to swim in the adult phase (Mianzan, Reference Mianzan and Boltovskoy1999; Oliveira and Migotto, Reference Oliveira and Migotto2007). These ctenophores are planktonic organisms and have ctene rows only as larvae. After their planktonic larvae settle down, the often brightly coloured benthic adults resemble nudibranchs or flatworms instead of ctenophores (Mills and Haddock, Reference Mills, Haddock and Carlton2007; Mills, Reference Mills2010). Benthic ctenophores have branched tentacles similar to those of cydippid species. This feature often allows observers to recognize these organisms as ctenophores (Mills, Reference Mills2010). The highly flattened and modified body are some of the features that differentiate benthic ctenophores from ‘typical’ planktonic forms (Mills and Haddock, Reference Mills, Haddock and Carlton2007; Oliveira and Migotto, Reference Oliveira and Migotto2007).
According to Mills (1998–present), there are approximately 200 species of Ctenophora, of which 50 species are benthic forms. Benthic ctenophores belong to the order Platyctenida, also known as platyctenes, and live on the bottom or as epibionts on other organisms such as algae or animals (Mills, Reference Mills1998–present). This order has five families, of which Coeloplanidae is the largest with two genera: Coeloplana with 33 species and Vallicula that only includes the conspicuous species of V. multiformis Rankin, Reference Rankin1956 (Mills, Reference Mills1998–present). Morphological identification of coeloplanid species is challenging because of their flexible shapes (Alamaru et al., Reference Alamaru, Hoeksema, van der Meij and Huchon2017). However, V. multiformis can be easily distinguished by the following characters: the anchor-shaped tentacle sheath, the blind ends of the canals near to the body margins and the presence of an oral groove connecting the base of the tentacle sheaths with the mount (Rankin, Reference Rankin1956). Its spatial distribution includes the Atlantic, Northeastern Pacific and Northern Indian waters where it has been observed on different substrates, including biotic and abiotic (Mills and Haddock, Reference Mills, Haddock and Carlton2007; Miyake et al., Reference Miyake, Wada, Adachi, Ohtsuka, Ikeda, Yonetani, Pagliawan, Metillo and Okoshi2019), predominantly in a sessile state, however, the species has also shown floating and crawling behaviour (Rankin, Reference Rankin1956).
A total of 33 ctenophore taxa have been identified in Mexican waters, of which seven have been recorded recently in the Mexican Caribbean Sea, and 12 taxa in jurisdictional waters of the Gulf of Mexico (see Puente-Tapia et al., Reference Puente-Tapia, Gasca, Schiariti and Haddock2021). This ctenophore diversity is represented only by planktonic forms, and so far, no benthic species had been reported for Mexico, lacking essential information such as the diversity and ecology for this group (Puente-Tapia et al., Reference Puente-Tapia, Gasca, Schiariti and Haddock2021). Hence, the present study records V. multiformis in Mexican waters for the first time increasing the known range of this gelatinous invertebrate group.
Material and methods
This record of the ctenophore V. multiformis is the result of an incidental finding during two different surveys carried out during daytime in the reef areas of the Cayo Arcas and Bonanza reefs (see description of the area below). These surveys were part of the project ‘Biodiversidad Marina de Yucatán-Universidad Nacional Autónoma de México’ (BDMY-UNAM; https://www.bdmy.org.mx), which characterizes the biodiversity of the Mexican Caribbean Sea and southern Gulf of Mexico along the Yucatan Peninsula in different marine and coastal environments.
Cayo Arcas reef complex is located at Campeche, Mexico, in the southern Gulf of Mexico, approximately 150 km offshore (20°12′18.44″N, 91°57′47″W) in a north-west orientation. This reef system belongs to the Campeche Bank, which is an underwater extension of the Yucatan Peninsula (Figure 1), and comprises fringing reefs, a cluster of three sand cays spread over an area of ~4 km × ~2.6 km (Robertson et al., Reference Robertson, Perez-España, Nuñez Lara, PucItza and Simões2016). In this reef complex, different habitats for molluscs, including algae and sponges, were collected manually (see Ortigosa and Simões, Reference Ortigosa and Simões2019) and stored in situ in plastic bags. Then, substrates were placed into white trays filled with seawater for posterior observation. They were left untouched and examined after 4–6 h. Vallicula multiformis was collected from the tray and photographed in situ.
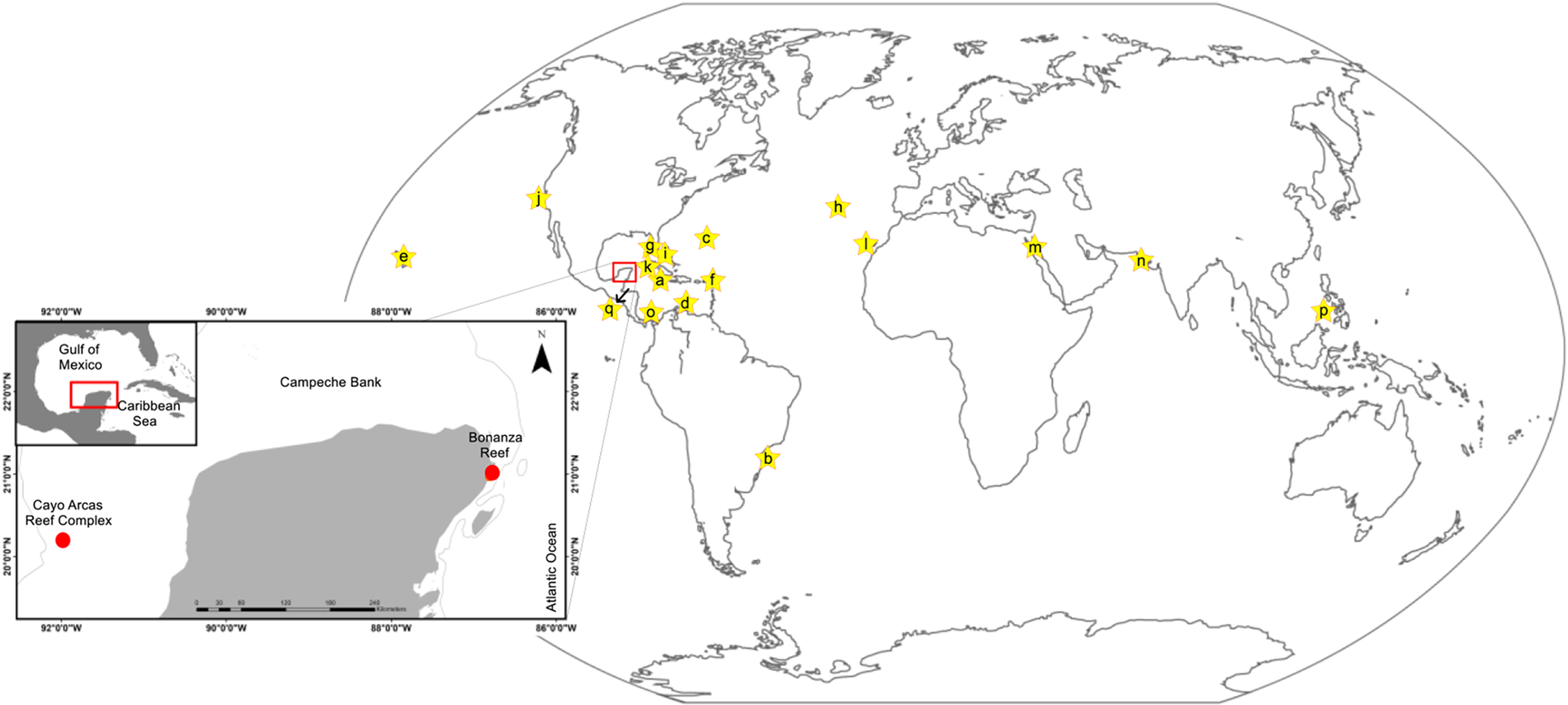
Figure 1. Map of the study area (Bonanza Reef, Quintana Roo, Caribbean Sea; Cayo Arcas Reef Complex, Campeche, Gulf of Mexico) and previous known worldwide distribution of Vallicula multiformis Rankin, Reference Rankin1956 (yellow stars): (a) Jamaica; (b) Brazil; (c) Bermuda; (d) Curazao; (e) Antigua and Saba Island; (f) USA (Florida); (g) USA (Hawaii); (h) Portugal (Madeira Island); (i) Bahamas; (j) USA (California); (k) Cuba; (l) Spain (Canary Islands); (m) Israel (Gulf of Aqada); (n) India (Boria and Adasaba Islands); (o) Panama; (p) Philippines (Palawan Island); (q) Honduras (Roatan Island). Order of the records is chronological. See the list of references of each record in Supplementary Table S1.
Bonanza Reef is located in coastal waters of Quintana Roo, Mexico in the Caribbean Sea (20°34′43.24″N, 86°29′17.26″W; NE coast of the Yucatan Peninsula), approximately 1 km offshore in a north–south orientation (Figure 1). It is mainly a marginal reef and, specifically in this area, the reef barrier is almost uninterrupted, wider and more developed (Ardisson et al., Reference Ardisson, May-Kú, Herrera-Dorantes and Arellano-Guillermo2011). Bonanza is a highly degraded reef with a predominance of erect macro algae and dead Acropora coral, with a length of ~1500 m and depth ranges between 1 and 5 m (Morillo-Velarde et al., Reference Morillo-Velarde, Briones-Fourzán, Álvarez-Filip, Aguíniga-García, Sánchez-González and Lozano-Álvarez2018). It belongs to the Mesoamerican Barrier Reef System (MBRS) and represents an important transitional area between the Caribbean Sea and the Gulf of Mexico (Kramer and Kramer, Reference Kramer and Kramer2002). In this reef, autonomous reef monitoring structures (ARMS), a standardized repeatable, passive and non-destructive sampling unit of the same area that mimics the reef structure in 3D (Zimmerman and Martin, Reference Zimmerman and Martin2004), were implemented.
The specimens collected were fixed in a 4% formaldehyde seawater solution and then placed in the invertebrate collection ‘Cnidaros del Golfo de México y Mar Caribe Mexicano (YUU-CC-254-11-001659)’ from Unidad Multidisciplinaria de Docencia e Investigación Sisal-Facultad de Ciencias-Universidad Nacional Autónoma de México (UMDI-FC-UNAM), Sisal Yucatan, Mexico. Taxonomic nomenclature and systematics followed Oliveira et al. (Reference Oliveira, Mianzan, Migotto and Marques2007), as well as the original morphological description of this species (see Rankin, Reference Rankin1956).
Results
Material examined
One specimen collected at 1.6 m depth in association with a green alga (Caulerpa sp.). Location: Cayo Arcas (Gulf of Mexico). Date: 19 August 2018. Maximal length observed: 8 mm along the tentacular axis, 4 mm wide (Figure 2A, B); one specimen collected at 4 m depth from an ARMS. Location: Bonanza Reef (Caribbean Sea). Date: 30 October 2020. Maximal length observed: 9 mm along the tentacular axis, 6 mm wide (Figure 2C).
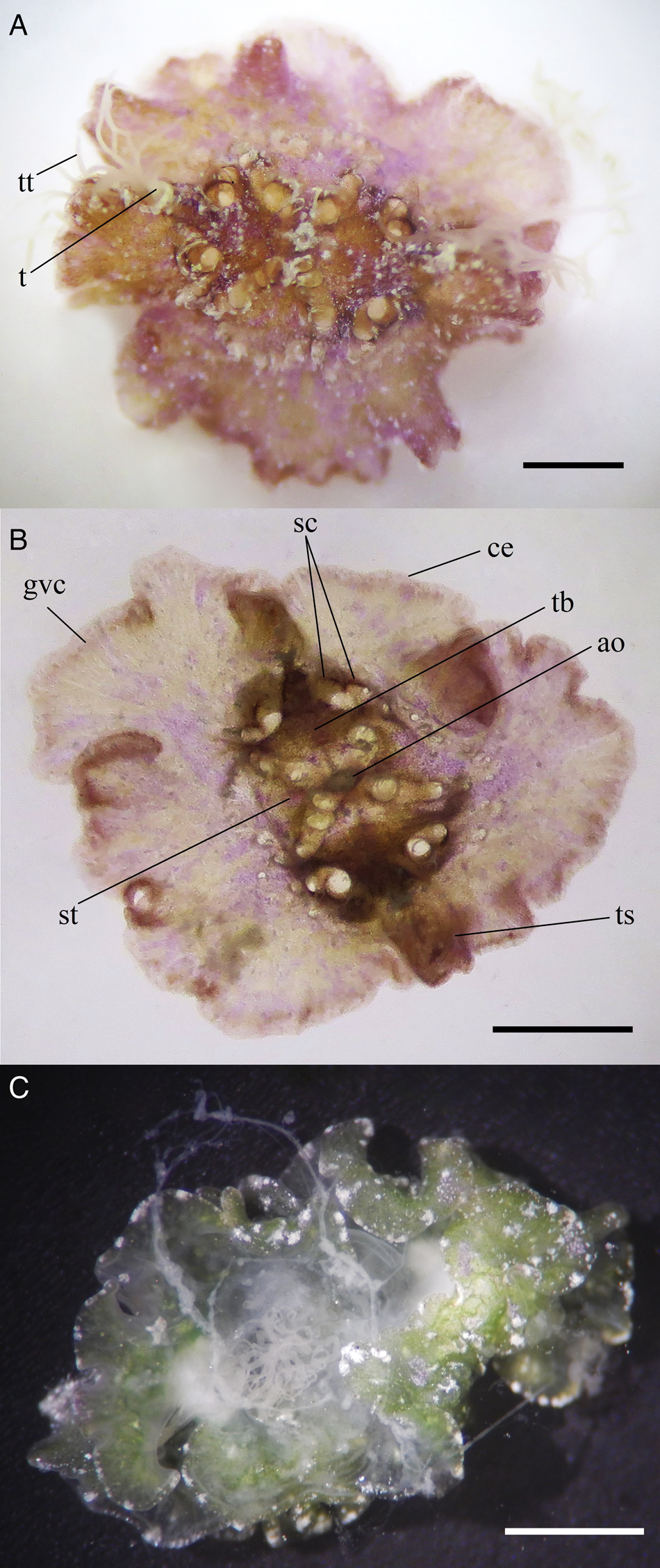
Figure 2. Vallicula multiformis Rankin, Reference Rankin1956 from Bonanza Reef (A, B, aboral view), Quintana Roo, Mexico (Caribbean Sea) and from Cayo Arcas Reef Complex (C, aboral view), Campeche, Mexico (Gulf of Mexico). ao, apical organ; ce, canals ending blindly near the margin; gvc, gastrovascular canal; sc, spherical chambers; st, stomach; t, tentacle; tb, tentacle bulb; tt, tentillae; ts, tentacular sheath; scale bars = 1 mm.
Morphological characteristics
The observed specimens exhibited a flattened body in the oral–aboral plane with folded-like body margins, assuming various forms and sizes depending on how they moved; the tentacle sheath (structure housing the tentacles) showed chimney-like tubular structures tilting upwards, which varied in length depending on the movement performed (Figure 2A). Tentacles were observed protruding and altering in shape and length from each tentacle's sheath, yet we could not determine their total lengths. Each tentacle displayed a large number of tentillae arising from the tentacle stem (Supplementary Video S1). Both the tentacles and tentillae were highly contractile, with a milky white colour; the specimens had eight spherical chambers, two on each side of the tentacle bulb; the apical organ was visible, as well as the reticulations of the gastrovascular system, mainly along the body margins, with canals ending blindly near the margins (Figure 2A, B); the stomach structure was visible mainly in the specimen collected from Bonanza Reef (Figure 2B); gonads were not identified; the aboral surface of the ctenophores had papillae characterized by variability in shape and position; small white granular pigments were concentrated along the perimeter of the specimen from Cayo Arcas (Figure 2C); the body colour was pale brown-pink (Figure 2A, B; specimen from Bonanza Reef) or pale green (Figure 2C; specimen from Cayo Arcas) with whitish markings or light colours, with some white, brown, pink or green pigments. In general, colours were more pronounced in the area immediately surrounding the apical organ, along the tentacular axis and around periphery.
Discussion
Most species identified in the order Platyctenida have a restricted geographical distribution, limited to their respective type localities (Prasade et al., Reference Prasade, Apte, Kale and Oliveira2015). However, V. multiformis has been observed in several regions worldwide, including tropical and subtropical marine waters (Figure 1). The records of V. multiformis in the present study confirm its incidence in the Caribbean Sea (Bonanza Reef) and extend its spatial distribution towards the southern Gulf of Mexico (Cayo Arcas).
Despite the different and continuous faunal monitoring (mainly benthic studies) performed by the project BDMY-UNAM in the Mexican Caribbean and the southern Gulf of Mexico, only two individuals of V. multiformis have been observed. The reproductive strategy of this species (Rankin, Reference Rankin1956; Glynn et al., Reference Glynn, Coffman, Primov, Renegar, Gross, Blackwelder, Martinez, Dominguez, Vanderwoude and Riegl2019) allows it to have high abundances during different seasons in some regions of its spatial distribution (e.g. Rankin, Reference Rankin1956; Eldredge and Miller, Reference Eldredge and Miller1995; Glynn et al., Reference Glynn, Coffman, Fuller, Moorhead, Williams, Primov, Fortson, Barrales and Glynn2017). Therefore, the number of specimens of this ctenophore and their occurrence on Mexican coasts possibly is underestimated due to its cryptic nature. Studies conducted in this region have not focused on benthic ctenophores; they are regularly confused with flatworms and nudibranchs because of their relative morphological similarity; consequently, the group is unnoticed frequently.
Due to the large geographical distribution of V. multiformis in the Caribbean Sea and adjacent areas (Figure 1), the occurrence of this species on Mexican waters could represent part of its natural distribution in this region of the Northwestern Atlantic. The distribution of this species could be assessed with studies focused on this group, for example, its frequency of occurrence and relative abundances during different seasons, as well as population genetic comparisons of different localities in order to determine if the organisms from Mexico are similar to other regions.
The ability of V. multiformis to live on a variety of substrates is an adaptive advantage and probably enables its large geographic distribution (Alamaru et al., Reference Alamaru, Brokovich and Loya2015). According to Glynn et al. (Reference Glynn, Coffman, Fuller, Moorhead, Williams, Primov, Fortson, Barrales and Glynn2017), the settlement densities of V. multiformis on artificial substrates indicate that biotic substrates are not essential for the attraction and colonization of inanimate surfaces by this species.
Morphological and ecological aspects
The maximum length of the specimens found in the present study (between 8 and 9 mm) is similar to values previously reported in different regions: 1–13 mm from Jamaican coasts (Rankin, Reference Rankin1956), 2–8 mm from Florida coasts (Glynn et al., Reference Glynn, Coffman, Vanderwoude, Martínez, Domínguez, Gross and Renegar2018) and 6–10 mm from the Indian waters (Prasade et al., Reference Prasade, Apte, Kale and Oliveira2015). However, Alamaru et al. (Reference Alamaru, Brokovich and Loya2015) identified specimens from the Israeli shore of the Gulf of Aqaba with a larger size range between 3 and 32 mm.
Vallicula multiformis occurs in shallow and sheltered biofouling communities, often adopting the colour of the substrate to which they are associated (Glynn et al., Reference Glynn, Coffman, Vanderwoude, Martínez, Domínguez, Gross and Renegar2018). We observed different coloration patterns between the two individuals collected. The specimen from the southern Gulf of Mexico (Cayo Arcas) had a similar pale green colour as the colour of the green algae Caulerpa sp. where it was found, while the specimen from the Mexican Caribbean Sea (Bonanza Reef) had a pale brown-pink colour, which is attributed to the brown pigment in the cells along the body. We also observed small red globules, which give a pinkish hue to the specimen when they are present in large numbers (Rankin, Reference Rankin1956).
The association between ctenophores and their hosts is an important aspect for species identification and may be used to differentiate species from the family Coeloplanidae: some species are host-specific and restricted to only one host (Alamaru et al., Reference Alamaru, Brokovich and Loya2015). Vallicula multiformis is a generalist species with a variety of hosts; it has been observed in association with green and brown algae, sponges, hydrozoan polyps, soft corals, bryozoans, sea urchins, holothurians, ascidians, rocks and artificial substrates (aquarium tanks, floats, rafts and ARMS) (see Rankin, Reference Rankin1956; Marcus, Reference Marcus1957; Wirtz, Reference Wirtz1998; Goodbody, Reference Goodbody2003; Mills and Haddock, Reference Mills, Haddock and Carlton2007; Moro et al., Reference Moro, Riera, Matsumoto and Ortea2010; Alamaru et al., Reference Alamaru, Brokovich and Loya2015; Prasade et al., Reference Prasade, Apte, Kale and Oliveira2015; Glynn et al., Reference Glynn, Coffman, Fuller, Moorhead, Williams, Primov, Fortson, Barrales and Glynn2017; Miyake et al., Reference Miyake, Wada, Adachi, Ohtsuka, Ikeda, Yonetani, Pagliawan, Metillo and Okoshi2019; present study). Since benthic ctenophores are epibionts on various algae and invertebrates in coral-reef areas worldwide, the biodiversity of this group could be expected to be high in the reef and coastal ecosystems (Alamaru et al., Reference Alamaru, Brokovich and Loya2015). Thus, V. multiformis probably is present in both the MBRS (Caribbean Sea) as in different coastal areas of the southern Gulf of Mexico with the presence of reef zones, such as the Veracruz Reef System (southern Gulf of Mexico).
In general, several gaps must still be addressed in the group of benthic ctenophores, for example, analyses to determine whether V. multiformis is a circumglobal species, a widely distributed species complex or, alternatively, an invasive species easily transported by marine currents, spread through shipping routes (either by ballast water, as part of fouling organisms or via aquarium trade) (see Eldredge and Miller, Reference Eldredge and Miller1995; Carlton and Eldredge, Reference Carlton and Eldredge2009; Alamaru et al., Reference Alamaru, Brokovich and Loya2015). Particularly for Mexico, it is necessary to conduct studies focused on this group to determine the real diversity, and the spatiotemporal variation of the occurrence and abundances. This study represents the first record of the occurrence of a species of benthic ctenophore in Mexican waters and it provides a baseline for the study of both the ecology and diversity of this type of ctenophores.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315423000401.
Acknowledgements
We are thankful to the team of the project BDMY-UNAM and leaders Dr Nuno Simões and Edlin Guerra-Castro. We thank Jonathan Santamaría for his support in sample processing; Dr Claudia Mills and Dr Peter Glynn helped with specimen identification and literature review. F. A. P.-T. thanks CONICET, Argentina, for the postdoctoral fellowship. D. O. had a postdoctoral fellowship from DGAPA-UNAM 2017-2018 during the survey to Cayo Arcas. L. A. P.-A. and X. G. V. had a doctoral fellowship from Consejo Nacional de Ciencia y Tecnología (CONACYT, CVU: 447073 and 564148). Also, we appreciate the comments and suggestions from the reviewers, which greatly improved this manuscript. This is a BDMY publication.
Author contributions
F. A. P.-T. and L. A. P.-A. wrote the first version of the manuscript. D. O. and X. G. V. carried out field sampling and collected the specimens. All authors contributed to the draft, provided critical feedback and helped shape the research. All authors read and approved the final manuscript.
Financial support
This work was supported by the Harte Research Institute and the Harte Charitable Foundation through grants given to Dr N. Simões. We also thank Dr María del Carmen Rivas of Puerto Morelos National Park (CONANP, request F00.9.DNPAPM/016/2020) for the facilities provided.
Competing interest
None.




