INTRODUCTION
Understanding the trophic ecology of a particular species is essential to determining its ecological role in marine ecosystems (Coll et al., Reference Coll, Navarro and Palomera2013; Ferretti et al., Reference Ferretti, Osio, Jenkins, Rosenberg and Lotze2013). Sharks and batoids are considered important components of marine food webs, often included in the top predator or mesopredator groups, although there are important differences in the diets among species (Cortés, Reference Cortés1999; Young et al., Reference Young, Olson, Ménard, Kuhnert, Duffy, Allain, Logan, Lorrain, Somes, Graham, Goñi, Pethybridge, Simier, Potier, Romanov, Pagendam, Hannides and Choy2015). In fact, the high diversity of feeding strategies makes the ecology of this entire marine group particularly complex to understand (Cortés, Reference Cortés1999). For this reason, the trophic role of these species is often unclear. To unravel this limitation, more trophic studies are essential, as they can help inform conservation strategies for threatened species (Ferretti et al., Reference Ferretti, Osio, Jenkins, Rosenberg and Lotze2013).
The Mediterranean Sea is considered a global hotspot of elasmobranch diversity, hosting ~7% of all elasmobranchs worldwide (Cavanagh et al., Reference Cavanagh and Gibson2007; Dulvy et al., Reference Dulvy, Fowler, Musick, Cavanagh, Kyne, Harrison, Carlson, Davidson, Fordham, Francis, Pollock, Simpfendorfer, Burgess, Carpenter, Compagno, Ebert, Gibson, Heupel, Livingstone, Sanciangco, Stevens, Valenti and White2014). However, most of the batoids and shark species in the Mediterranean Sea have declined in abundance and distribution, mainly due to human impacts (Ferretti et al., Reference Ferretti, Worm, Britten, Heithaus and Lotze2010; Coll et al., Reference Coll, Navarro and Palomera2013). In fact, around 40% of the elasmobranchs are considered threatened in the Mediterranean Sea by regional assessments of the International Union for Conservation of Nature (IUCN) (Abdul Malak et al., Reference Abdul Malak, Livingstone, Pollard, Polidoro, Cuttelod, Bariche, Bilecenoglu, Carpenter, Collette, Francour, Goren, Kara, Enric, Papaconstantinou and Tunesi2011; Bradai et al., Reference Bradai, Saidi and Enajjar2012).
In comparison with other Mediterranean areas, research focusing on elasmobranchs inhabiting the Levantine Sea (eastern Mediterranean Sea) is very limited (Cavanagh et al., Reference Cavanagh and Gibson2007), even though these waters host endemic, threatened and rare elasmobranchs. This is the case of the threatened batoids Gymnura altavela (spiny butterfly ray), with a vulnerable status, and the Mediterranean endemic Raja asterias (Mediterranean starry ray) and Raja clavata (thornback ray), both with a near threatened status based on the IUCN Red List (Vooren et al., Reference Vooren, Piercy, Snelson, Grubbs, Notarbartolo di Sciara and Serena2007; Serena et al., Reference Serena, Abella, Walls and Dulvy2015; Ellis et al., Reference Ellis, Dulvy and Serena2016). In the Levantine Sea, these three batoid fishes are highly impacted by the demersal fisheries operating in coastal and deep-sea waters (Dalyan, Reference Dalyan2012; Yeldan et al., Reference Yeldan, Avşar, Mavruk and Manaşırlı2013; Yemisken et al., Reference Yemisken, Dalyan and Eryilmaz2014).
Regarding their trophic habits, previous studies based on stomach contents conducted in the western and central Mediterranean Sea indicated that these three batoids act as mesopredators in the ecosystem, exploiting a wide variety of resources including crustaceans, demersal fish and cephalopods (Cuoco et al., Reference Cuoco, Mancusi and Serena2005; Romanelli et al., Reference Romanelli, Colasante, Scacco, Consalvo, Finoia and Vacchi2007; Valls et al., Reference Valls, Quetglas, Ordines and Moranta2011; Santic et al., Reference Santic, Rada and Pallaoro2012; Navarro et al., Reference Navarro, Coll, Preminger and Palomera2013). Although stomach content methodology permits a high level of taxonomic resolution in the identification of prey, batoids and sharks show a high frequency of empty stomachs. Moreover, the prey species that are often found in the stomachs are those of slower digestion rates, which could cause biases in the diet estimation (Hyslop, Reference Hyslop1980). In addition, this conventional method requires a large number of stomachs to quantify diet. This can be difficult to obtain, especially for endangered species. The use of stable isotopes of nitrogen (δ15N) and carbon (δ13C) has been used as a complement to stomach content analysis to study the trophic ecology of elasmobranch species (e.g. Shiffman et al., Reference Shiffman, Gallagher, Boyle, Hammerschlag-Peyer and Hammerschlag2012). This approach is based on the fact that δ15N and δ13C values are transformed from dietary sources to consumers in a predictable manner (Shiffman et al., Reference Shiffman, Gallagher, Boyle, Hammerschlag-Peyer and Hammerschlag2012). δ15N values show a predictable increase from one trophic level to the next (Jennings et al., Reference Jennings, Reñones, Morales-Nin, Polunin, Moranta and Coll1997; Layman et al., Reference Layman, Araujo, Boucek, Hammerschlag-Peyer, Harrison, Jud, Matich, Rosenblatt, Vaudo, Yeager, Post and Bearhop2012). δ13C values show little change due to trophic transfer, but are useful indicators of dietary sources of carbon (Layman et al., Reference Layman, Araujo, Boucek, Hammerschlag-Peyer, Harrison, Jud, Matich, Rosenblatt, Vaudo, Yeager, Post and Bearhop2012).
In this study, we investigated the feeding ecology (diet, trophic width and trophic position) of three batoids (G. altavela, R. asterias and R. clavata) coexisting in Iskenderun Bay (north-eastern Levantine Sea, Mediterranean Basin) by combining stomach contents and stable isotope analyses. Our study provides new insights into the ecological role of these three endangered batoid species in the Levantine Sea.
MATERIALS AND METHODS
Study area and sampling procedure
The north-east Levantine Sea (Figure 1) has a wide continental shelf and shows high marine productivity influenced by local wind effects, upwelling movements and rich terrestrial nutrient inputs from the Asi River (Polat & Piner, Reference Polat and Piner2002). The area includes a high richness of marine species within the eastern Mediterranean Sea (Bilecenoğlu, Reference Bilecenoğlu2016). The oceanographic conditions, such as the environmental conditions similar to tropical and sub-tropical regions, has promoted the colonization by invasive species from the Red Sea (Bilecenoğlu, Reference Bilecenoğlu2016).
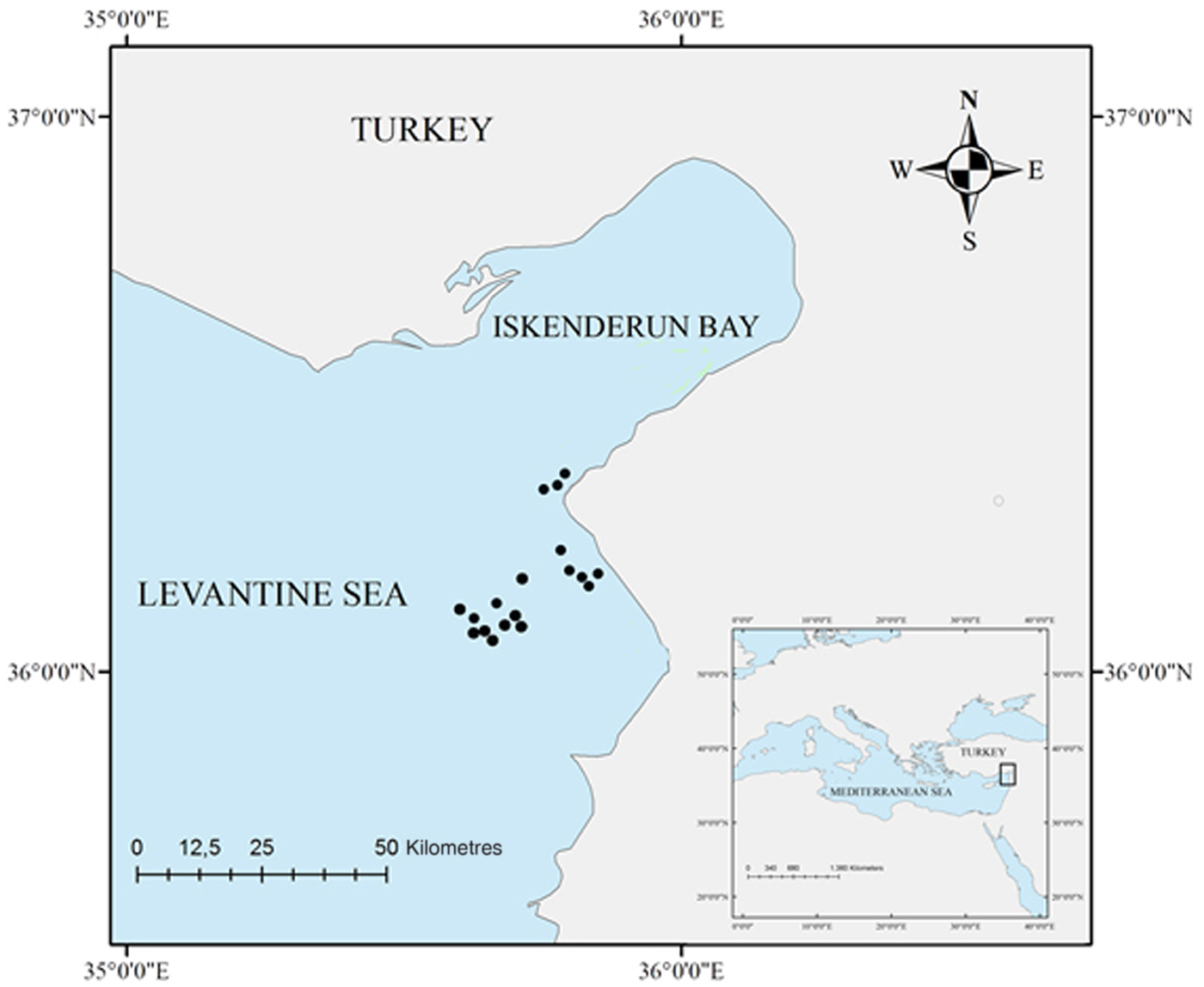
Fig. 1. Study area (North-eastern Levantine Sea, eastern Mediterranean Sea), indicating the sampling locations (black points).
A total of 13 G. altavela, 46 R. asterias and 26 R. clavata individuals were collected between September 2014 and April 2016 at a depth ranging from 33 to 450 m, by commercial trawl vessels in the Iskenderun Bay (Figure 1). All individuals were accidentally captured as by-catch of the fishing fleet. Individuals were taken to the lab in a freezer where body size (disc width length; DW, to the nearest mm) and weight (nearest g) were recorded.
Stomach content analysis
All prey items presented in the stomachs were identified at the lowest taxonomic levels (copepods, decapods, cephalopods and teleosts). Per cent of number (%N), weight (%W) and frequency of occurrence (%F) of prey items were calculated and these values were utilized for calculating the Index of Relative Importance (IRI) of each prey item (IRI = %F(%N + %W)). The IRI was standardized using the formula: %IRI = (IRI/ΣIRI) × 100 (Cortés, Reference Cortés1997). The vacuity index (v; the percentage of empty stomachs) and the percentage of fullness of stomachs (Fullness %) were also calculated. Levin's and Pianka's measures were used to determine niche breadth (Bi) with the standardized niche breath (BA), and niche overlap between the three batoid fishes (Colwell & Futuyma, Reference Colwell and Futuyma1971). Prey-specific abundance was calculated according to the following: P i = (ΣS i /ΣS t) × 100, where P i is the prey-specific abundance of prey i, S i is the stomach contents (number) including prey i, and S t is the total stomach contents among those individuals with prey in their stomach (Amundsen et al., Reference Amundsen, Gabler and Staldvik1996).
Stable isotope analysis
We obtained a muscle sample from the pectoral fin of seven spiny butterfly rays, seven Mediterranean starry rays and nine thornback skates. Before stable isotope analysis, we extracted lipid from muscle samples using a chloroform-methanol solution (Kim & Koch, Reference Kim and Koch2011). Samples were subsequently freeze-dried and powdered and 0.28–0.4 mg of each sample was packed into tin capsules. Isotopic analyses were performed at the Stable Isotopes Laboratory at the Estación Biológica de Doñana CSIC (Seville, Spain). Samples were combusted at 1020°C using a continuous flow isotope ratio mass spectrometry system (Thermo Electron) by means of a Flash HT Plus elemental analyser coupled to a Delta-V Advantage isotope ratio mass spectrometer. Stable isotope ratios were expressed in the standard δ-notation (‰) relative to Vienna Pee Dee Belemnite (δ13C) and atmospheric N2 (δ15N). Based on laboratory standards, the measurement error was ±0.1 and ±0.3 for δ13C and δ15N, respectively (Cabana & Rasmussen, Reference Cabana and Rasmussen1996).
As a measure of trophic width, for each species a Bayesian isotopic ellipse area (SEA) was calculated from the stable isotope values (Jackson et al., Reference Jackson, Inger, Parnell and Bearhop2011). This metric represents a measure of the total amount of the isotopic niche exploited by a particular predator and is thus a proxy for the extent of trophic diversity (or trophic width) exploited by the species (high values of isotopic standard ellipse areas indicate high trophic width). This metric uses multivariate ellipse-based Bayesian metrics. Bayesian inference techniques allow for robust statistical comparisons between datasets with different sample sizes. Isotopic standard ellipse areas were calculated using the routine Stable Isotope Bayesian Ellipses incorporated in the SIAR library (SIBER, Jackson et al., Reference Jackson, Inger, Parnell and Bearhop2011).
Trophic position
The trophic position (TP) of each species was estimated by using isotopic values (TPSIA) and stomach content analysis (TPstomach). TPSIA was performed according to Zanden & Rasmussen (Reference Zanden and Rasmussen2001):
where δ15 N consumer is the value for each batoid species and δ15 N basal is that of the crab, Monodaeus couchii (7.1‰) sampled from the north-eastern Levantine Sea. We used 1.95 for Δ15 N values (Hussey et al., Reference Hussey, Brush, McCarthy and Fisk2010), defined as the trophic enrichment factor between organism and diet.
TPstomach was calculated using the following equation: TL j = 1 + Σn j –1 IRI% * TP i , where j is the predator of prey i, IRI% is the fraction of prey i in the diet of predator j, and TL i is the trophic position of prey i (Cortés, Reference Cortés1999). Trophic positions of prey categories were based on Ebert & Bizzarro (Reference Ebert and Bizzarro2007).
Statistical analysis of stomach content data
Data analysis was performed with multivariate techniques (PERMANOVA). The diets of the three batoid species were analysed using the Bray–Curtis resemblance matrix of log(x + 1) transformed, with prey abundance data. The comparison similarities of prey groups among the three batoids were determined by SIMPER and appeared as vector overlay on the principal coordinates analysis (PCO) plot by PRIMER v6 (Clarke & Warwick, Reference Clarke and Warwick2001). One-way analysis of covariance (ANCOVA) was used to reveal relationships between body size and the fullness index in stomach contents with SPSS 21 software.
RESULTS
Stomach content analysis
A total of 85 individual stomachs were analysed belonging to three batoids. We found that 71 of these individuals had food in their stomachs (coefficient of vacuity: 32% for R. asterias, 15% for R. clavata and 31% for G. altavela). We identified 15 different prey species belonging to three different taxonomic groups (Table 1). The minimum average of percentage of fullness was estimated at 14% for the spiny butterfly ray and 43% for the thornback skate and Mediterranean starry ray. There were no significant differences between the fullness index and body size in any of the three batoids (P > 0.05).
Table 1. Diet composition of Gymnura altavela, Raja asterias and Raja clavata in the Iskenderun Bay (DW, disc width; TL, trophic level estimated from stomach contents; N, number of stomach; %FO, frequency of occurrence; %N, percentage in number; %W, percentage in mass; %IRI, index of relative importance of prey).

Although teleosts were the main prey group for all three batoids, we found significant differences in the diet composition (PERMANOVA tests, Pseudo-F 2,20 = 28.01; P < 0.001). In particular, pairwise tests indicated that stomach contents differed between G. altavela and both R. asterias and R. clavata. The PCO analysis showed that the horizontal axes explain separation with 57.7% total variation because of the contribution of cephalopods and decapods to the diet of the batoids. The vertical axes explained separation with 29.8% total variation in accordance with the contribution of the teleost species to the diets (Figure 2). While the teleost group was common in all batoids, the main differences among the batoid species were found in decapod and cephalopod groups.
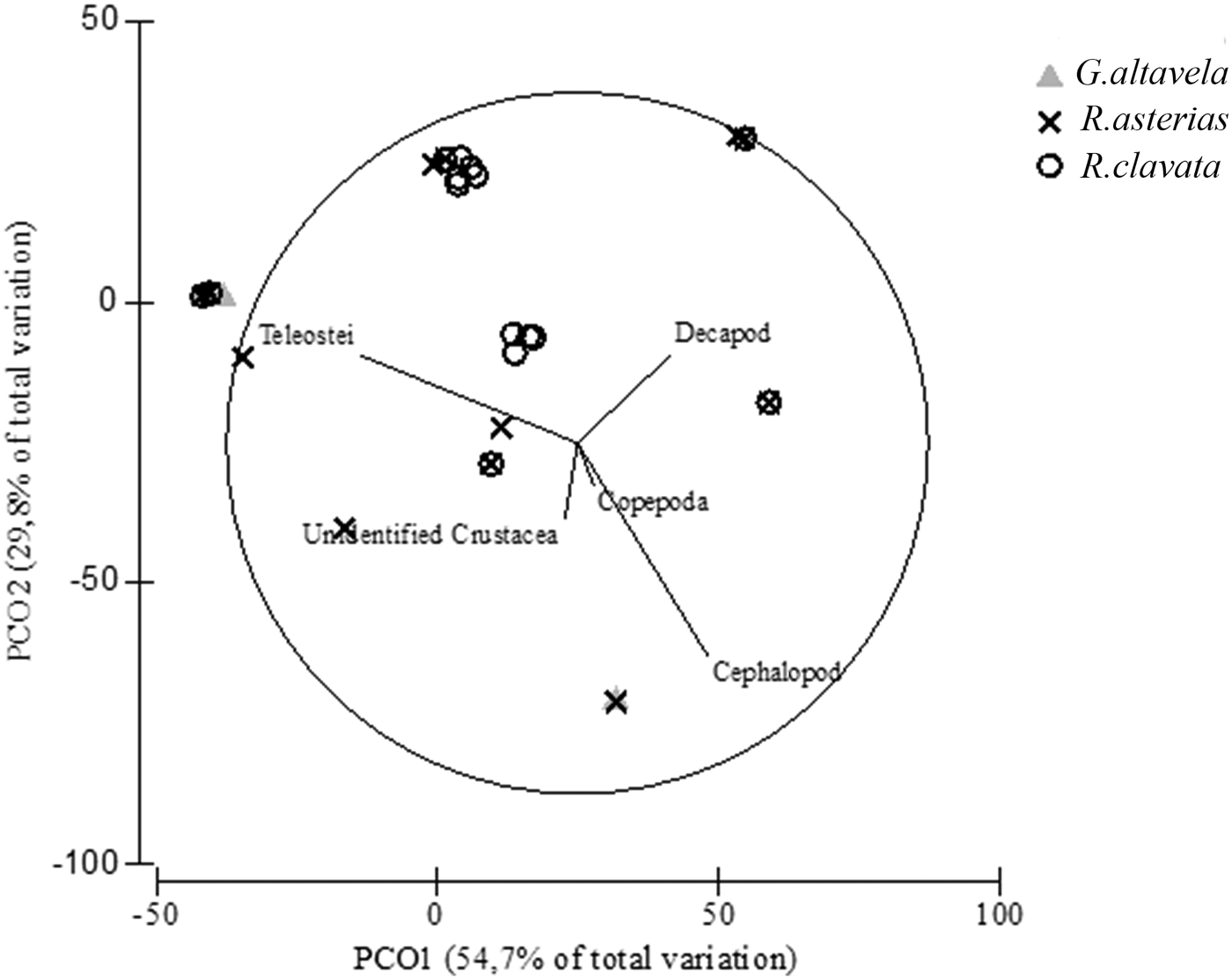
Fig. 2. Principal coordinates analysis of stomach contents from G. altavela, R. asterias and R. clavata from the north-eastern Levantine Sea (Mediterranean Sea).
Champsodon sp. was a common prey fish species in the stomach of G. altavela and R. clavata, whereas Equulites klunzingeri (IRI = 21.13%) was only found in the stomach of G. altavela (Table 2). In addition, Chlorophthalmus agassizi was found in the stomachs of both R. asterias and R. clavata. Argentina sphyraena (IRI = 0.2%), Bregmaceros atlaticus (IRI = 0.4%) and Trachurus sp. (IRI = 0.5%) were identified only in the stomach contents of R. asterias. Decapoda was the second main prey group for R. asterias and R. clavata (IRI = 18.68% and IRI = 44.40%, respectively). Copepods were only found in the stomach of R. asterias (IRI = 1.7%). Although it was somewhat common to find cephalopods in the batoid stomachs, they were not represented in a high percentage of the stomach contents (IRI % between 2.15 and 10.64).
Table 2. Sample size (N) and mean and standard deviation of isotopic values and trophic level estimated with δ15N values (TLSIA) of three batoids in the Iskenderun Bay (north-eastern Mediterranean Sea).

Regarding the niche width, G. altavela showed lower values (B i : 1.1, BA: 0.1), followed by R. asterias (B i : 3.32, BA: 0.8) and R. clavata (B i : 3.25, BA: 0.8). Diet overlap was lowest between G. altavela and R. clavata (0.46), and highest between R. asterias and R. clavata (0.98). The relationship between prey specific abundance and prey occurrence confirms a specialist feeding strategy on teleosts for G. altavela (Figure 3). In contrast, R. asterias and R. clavata displayed generalist feeding strategies.
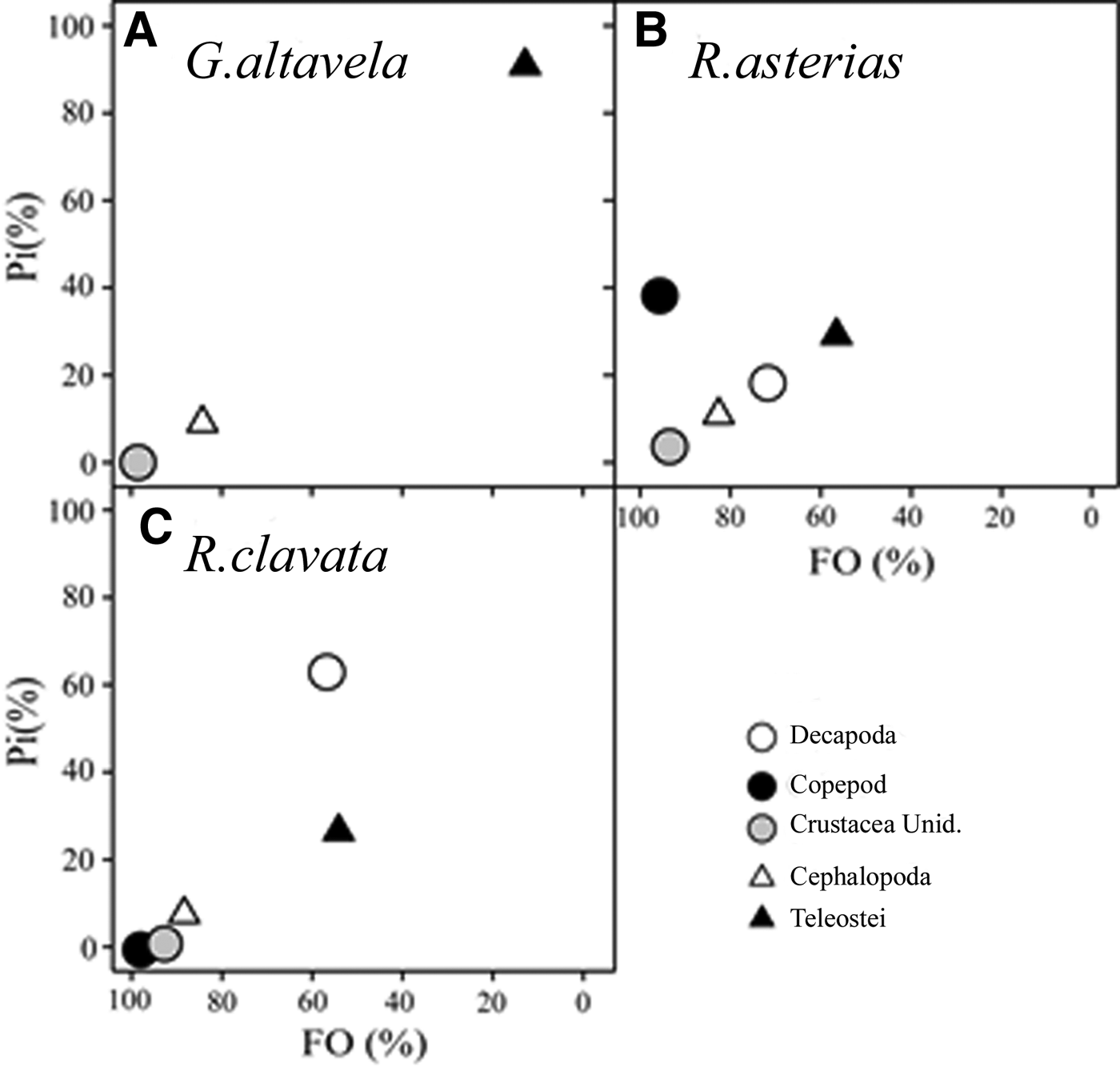
Fig. 3. Graphical representation of the feeding strategy of G. altavela (A), R. asterias (B) and R. clavata (C) from the north-eastern Levantine Sea (Mediterranean Sea): prey-specific abundance (Pi %) plotted against mean frequency of occurrence (%FO) of the different prey groups.
Stable isotope results
Combined values of stable nitrogen and carbon differed among batoid species (δ15N, F 2,21 = 73.22, P < 0.001; δ13C, F 2,21 = 24.38, P < 0.001). Specifically, R. asterias and R. clavata did not differ in their stable nitrogen and carbon values (Tukey post hoc tests, all P > 0.05; Table 2, Figure 4) but showed lower values of stable isotopes than G. altavela (post hoc test, P < 0.05; Table 3, Figure 4). The isotopic niche width based on the Standard Ellipse Area (SEA) clearly differed between batoid species (Figure 4), with the highest values for the thornback skate (SEA = 0.91‰), followed by G. altavela (SEA = 0.91‰) and R. asterias (SEA = 0.41‰) (Figure 4).

Fig. 4. Mean and standard deviation of δ13C, δ15N and trophic level values of G. altavela, R. asterias and R. clavata from the north-eastern Levantine Sea (Mediterranean Sea). The Bayesian standard ellipse areas are also indicated.
Table 3. Main prey groups in the diet of Gymnura altavela, Raja asterias and Raja clavata from the Mediterranean Sea. NW, north-western; SC, south-central; C, central; W, western; SE, south-east.

Trophic level
The trophic position estimated from stomach contents (TPstomach) varied between 3.88 and 4.24 among the three batoids, with G. altavela having a higher value than R. asterias and R. clavata, which occupied a very similar trophic position. When we estimated the trophic level from nitrogen isotope values, we found that absolute values differed from those estimated by stomach contents, but the relative position of the three studied species remained similar (Table 2).
DISCUSSION
In this study, the trophic ecology of three batoids (G. altavela, R. asterias and R. clavata) inhabiting the Levantine Sea (East Mediterranean Sea) was studied by combining stomach contents and isotope analyses. Stomach content results provide a snapshot of the diet of each species, and isotopic values identify the trophic width and trophic level integrating a long-term view (Peterson & Fry, Reference Peterson and Fry1987; Kim & Koch, Reference Kim and Koch2011; Navarro et al., Reference Navarro, López, Coll, Barría and Sáez-Liante2014). Based on the results of both stomach contents and stable isotopes, we found clear differences in the trophic habits among these three demersal predators.
Stomach contents revealed that the diet of G. altavela was mainly composed of fish prey, a result that agrees with the very few studies conducted previously in this species in Mediterranean waters (Table 3; Neifar et al., Reference Neifar, Euzet and Ben Hassine2002; Psomadakis et al., Reference Psomadakis, Dalù, Scacco and Vacchi2008; Barría et al., Reference Barría, Coll and Navarro2015). This indicates that this species is a predator with clear preferences for fish. Although R. asterias and R. clavata also included fish in their diet, crustaceans were important prey as well for these species, contributing to the diet in the same proportion as fish. These results contrast with those from other locations in the Mediterranean, where the diet of these two rajidae species were composed mainly by crustaceans (Kabasakal, Reference Kabasakal2001; Vannucci et al., Reference Vannucci, Mancusi, Serena, Cuoco and Volani2006; Valls et al., Reference Valls, Quetglas, Ordines and Moranta2011; Navarro et al., Reference Navarro, Coll, Preminger and Palomera2013; Eronat & Özaydın, Reference Eronat and Özaydın2015; Fatimetou & Younes, Reference Fatimetou and Younes2016). For example, Navarro et al. (Reference Navarro, Coll, Preminger and Palomera2013) found that crabs were the dominant prey for R. asterias in the western Mediterranean Sea. In the Ligurian Sea and Tyrrhenian Sea, similar results were found with R. asterias. Goneplax rhomboides and Liocarcinus sp. were reported mostly in stomach content of R. asterias from shallow water (Cuoco et al., Reference Cuoco, Mancusi and Serena2005; Romanelli et al., Reference Romanelli, Colasante, Scacco, Consalvo, Finoia and Vacchi2007). Yeldan (Reference Yeldan2005) showed that crustacean species were the main prey in the diet of R. asterias along the east coast of the Iskenderun Bay (North Levantine Sea). The current study differs from Yeldan (Reference Yeldan2005) in its sampling area. Yeldan (Reference Yeldan2005) sampled the individuals in coastal waters, where the availability of crustaceans is high. Our samples of Raja spp. were captured mostly from deeper waters. Discrepancies in the diet of R. clavata between our study and those carried out previously are probably due to geographic and depth differences reported for this batoid (Kabasakal, Reference Kabasakal2001; Vannucci, Reference Vannucci, Mancusi, Serena, Cuoco and Volani2006; Valls et al., Reference Valls, Quetglas, Ordines and Moranta2011; Eronat & Özaydın, Reference Eronat and Özaydın2015). For instance, Eronat & Özaydın (Reference Eronat and Özaydın2015) indicated the dominant occurrence of crustaceans in the diet of R. clavata between 120 and 350 m in the Aegean Sea, while Valls et al. (Reference Valls, Quetglas, Ordines and Moranta2011) showed that the contribution of teleosts was much more relevant for this species in deeper waters. In our study, the relative contribution of crustaceans and teleosts was nearly the same.
The existence of interspecific differences in teeth morphology could explain differences in the diet (McEachran & Capapé, Reference McEachran, Capapé, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1984; Jacobsen & Bennett, Reference Jacobsen and Bennett2013). The presence of crushing teeth plates in the two Raja spp. probably confers a greater capacity to crush the carapace of crustaceans, whereas the cuspidate teeth of G. altavela facilitate the capture of fish (Vannucci et al., Reference Vannucci, Mancusi, Serena, Cuoco and Volani2006; Motta & Huber, Reference Motta, Huber, Carrier, Musick and Heithaus2012; Ellis et al., Reference Ellis, Dulvy and Serena2016). Based on the principle of competitive exclusion, we expect that competing predators coexisting in the same waters segregate their exploitation of trophic resources (e.g. Papastamatiou et al., Reference Papastamatiou, Wetherbee, Lowe and Crow2006; Follesa et al., Reference Follesa, Mulas, Cabiddu, Porcu, Deiana and Cau2010; Albo-Puigserver et al., Reference Albo-Puigserver, Navarro, Coll, Aguzzi, Cardona and Sáez-Liante2015). For this reason, the three batoids partially segregate their main trophic resources as a mechanism that allows coexistence in the demersal habitat.
As expected from the stomach content results, interspecific differences in the isotopic values and trophic levels were found. In particular, G. altavela was isotopically segregated from R. asterias and R. clavata, showing a lower isotopic trophic width and higher trophic level. The trophic width estimated from SEAs was larger for G. altavela and R. clavata in comparison to R. asterias. Distribution of R. clavata shows variety from shallow to deep water in the area. This could be a result of the more generalized feeding strategy of R. clavata. On the other hand, previous studies on the feeding ecology of R. asterias show its specialized feeding strategy on crustacean species (Barría et al., Reference Barría, Coll and Navarro2015). The diversity richness of the coastal area in which G. altavela is mainly distributed (Emre Yemisken, unpublished data) probably explains the high trophic width of this species. Based on the trophic position of the species, both methodologies (stomach contents and isotopic values) revealed that G. altavela was at a higher position than the other two species. This pattern was previously found within demersal food webs in the western Mediterranean Sea where G. altavela shows a higher trophic position than coexisting batoids (Valls et al., Reference Valls, Quetglas, Ordines and Moranta2011; Barría et al., Reference Barría, Coll and Navarro2015), probably related to its large body size.
Although we expected a similar estimation of trophic position using stable isotope analysis (SIA) and stomach contents, we found differences between the methods in both Rajidae species. The estimation of trophic level from stable isotopes was lower than from stomach contents. Differences between TPsia and TPstomach would be expected considering that the estimated trophic levels from isotopic data are vulnerable to the basic assumption of which basal sources are used (Olin et al., Reference Olin, Hussey, Grgicak-Mannion, Fritts, Wintner and Fisk2013). Discrepancies between the methodologies (TPsia and TPstomach) revealed the need for caution when values of trophic levels are compared (Albo-Puigserver et al., Reference Albo-Puigserver, Navarro, Coll, Aguzzi, Cardona and Sáez-Liante2015). However, differences observed in the trophic position between the two methods in this study might be explained by long-term and short-term prey preference differences of Rajidae species in the region. When resources are restricted in the ecosystem, sometimes species may adapt and change their feeding behaviour after a while in the area. Although stomach content results have shown teleost and shrimp preferences in feeding behaviour, prey availability may not be sustainable on the same prey.
In conclusion, this study presents new information regarding the feeding ecology of three endangered batoids (G. altavela, R. asterias and R. clavata) in the Levantine Sea. The results indicate differences in the diet between species, showing a clear feeding preference for teleosts in the case of G. altavela and a diet composed of fish and crustaceans in the case of R. asterias and R. clavata. These results can be used by managers to conduct an appropriate assessment and inform conservation strategies for these species.
ACKNOWLEDGEMENTS
We thank Suna Tüzün, Onur Gonual and Mert Kesiktas for their help during the sampling and laboratory process and special thanks to Susana Carrasco during the stable isotope analysis at Laboratory of Estación Biológica de Doñana CSIC (Seville, Spain).
FINANCIAL SUPPORT
This study was partially funded by Istanbul University (project no: 42822) and TUBITAK 2214A (PhD student international scholarships programme).









