INTRODUCTION
Patients with epilepsy are at risk for several problems, which can affect their life quality and social competences more than the seizures themselves.
Among these issues, cognitive and neuropsychological impairments represent a very impacting factor, particularly in those patients who show structural etiology (Rudzinski & Meador, Reference Rudzinski and Meador2013; Witt & Helmstaedter, Reference Witt and Helmstaedter2012). For example, the reported incidence of cognitive dysfunctions in children with focal cortical dysplasia ranges from 50% to 80% (Korman et al., Reference Korman, Krsek, Duchowny, Maton, Pacheco-Jacome and Rey2013; Krsek et al., Reference Krsek, Maton, Korman, Pacheco-Jacome, Jayakar, Dunoyer and Duchowny2008). Furthermore, other structural epilepsies, such as temporal lobe epilepsy, associated with mesiotemporal sclerosis, have been associated with high rates of cognitive impairments (Allone et al., Reference Allone, Lo Buono, Corallo, Pisani, Pollicino, Bramanti and Marino2017). Furthermore, recent findings have shown in pediatric temporal lobe epilepsy an association of executive functions with depression (Schraegle, Nussbaum, & Titus, Reference Schraegle, Nussbaum and Titus2018). However, impairment of overall cognitive function or isolated neuropsychological difficulties, such as visual perception, attention, and memory, have been reported even in self-limited focal epilepsies in childhood, with no MRI evidence of brain lesions, easily controlled seizures, and good prognosis (Deonna et al., Reference Deonna, Zesiger, Davidoff, Maeder, Mayor and Roulet2000; Pal et al., Reference Pal, Ferrie, Addis, Akiyama, Capovilla, Caraballo and Koutroumanidis2016). The risk of both pervasive and specific neuropsychological impairments is also reported in idiopathic generalized epilepsy (Henkin et al., Reference Henkin, Sadeh, Kivity, Shabtai, Kishon-Rabin and Gadoth2005; Loughman, Bowden, & D’Souza, Reference Loughman, Bowden and D’Souza2014). In fact, both self-limited focal epilepsies and idiopathic generalized epilepsies have a complex and polygenic genetic background that could be in a relationship and in common with cognitive impairment (Lesca et al., Reference Lesca, Rudolf, Labalme, Hirsch, Arzimanoglou, Genton and Szepetowski2012; Ratcliffe et al., Reference Ratcliffe, Wandschneider, Baxendale, Thompson, Koepp and Caciagli2020). Finally, the most severe condition is represented by the developmental and epileptic encephalopathies with neonatal to childhood-onset that are associated with drug-resistant epilepsy and significant cognitive impairment.
Among the possible neuropsychological disturbances, executive and attention problems are highly reported and they are a common feature among the different epilepsies types (Conant, Wilfong, Inglese, & Schwarte, Reference Conant, Wilfong, Inglese and Schwarte2010; Culhane-Shelburne, Chapieski, Hiscock, & Glaze, Reference Culhane-Shelburne, Chapieski, Hiscock and Glaze2002; D’Agati, Cerminara, Casarelli, Pitzianti, & Curatolo, Reference D’Agati, Cerminara, Casarelli, Pitzianti and Curatolo2012) (Witt & Helmstaedter, Reference Witt and Helmstaedter2012; for a review, see MacAllister, Vasserman, Rosenthal, & Sherman, Reference MacAllister, Vasserman, Rosenthal and Sherman2014). However, the cause of this association deserves further investigation. Executive functions represent a constellation of cognitive skills that drive goal-oriented behavior and are critical to the ability to adapt to an ever-changing world. Up to 50% of children with epilepsy demonstrate executive dysfunctions (Campiglia et al., Reference Campiglia, Seegmuller, Le Gall, Fournet, Roulin and Roy2014; Høie et al., Reference Høie, Sommerfelt, Waaler, Alsaker, Skeidsvoll and Mykletun2008; Parrish et al., Reference Parrish, Geary, Jones, Seth, Hermann and Seidenberg2007; Slick, Lautzenhiser, Sherman, & Eyrl, Reference Slick, Lautzenhiser, Sherman and Eyrl2006). Deficits can persist or worsen over time (Bailet & Turk, Reference Bailet and Turk2000; Masur et al., Reference Masur, Shinnar, Cnaan, Shinnar, Clark, Wang and Glauser2013; Piccinelli et al., Reference Piccinelli, Beghi, Borgatti, Ferri, Giordano, Romeo and Balottin2010) with impact on academic (Fastenau et al., Reference Fastenau, Shen, Dunn, Perkins, Hermann and Austin2004; Høie et al., Reference Høie, Sommerfelt, Waaler, Alsaker, Skeidsvoll and Mykletun2008) and social functioning (Nassau & Drotar, Reference Nassau and Drotar1997). In the same way, attention deficits have been found to have significant implications on children’s global functioning in the presence of epilepsy. Interestingly, it has shown that inattention is specifically implicated in academic underachievement (Seidenberg et al., Reference Seidenberg, Beck, Geisser, O’Leary, Giordani and Berent1988) and that attention appears to be the only variable that predicts academic performance in children with epilepsy among memory abilities, self-esteem, and socioeconomic status and after controlling for intelligence (Williams et al., Reference Williams, Phillips, Griebel, Sharp, Lange, Edgar and Simpson2001). ADHD is also overrepresented among children with epilepsy in comparison to the general population (Dunn & Kronenberger, Reference Dunn and Kronenberger2005); furthermore, epileptic patients with ADHD meet the usual criteria for inattentive-type ADHD (Dunn & Kronenberger, Reference Dunn and Kronenberger2005; Gascoigne et al., Reference Gascoigne, Smith, Barton, Webster, Gill and Lah2017).
With the aim to provide an overview of neuropsychological impairments in childhood epilepsies, with a particular focus on attention and executive functions, we evaluated the performances of children suffering from the three main categories of epilepsy, such as focal/structural epilepsies, focal self-limited epilepsies, and generalized epilepsies. Further, we analyzed the longitudinal evolution of the neuropsychological profile in these three groups of patients.
METHODS
Participant and Eligibility Criteria
We retrospectively identified all patients undergoing neuropsychological evaluation at the Pediatric Neurology and Neurophysiology Unit of the Department of Women’s and Children’s Health, University Hospital of Padua (Italy) between September 2012 and February 2018. Among all the identified patients, we considered only those with complete and standardized evaluations. Subsequently, we selected those patients with at least two subsequent neuropsychological assessment. In order to increase the comparability between neuropsychological tests, we selected the patients aged 6 to 16 years.
Children were grouped based on the three following categories: focal/structural epilepsies, so-called focal self-limited, and idiopathic generalized epilepsies (Scheffer et al., Reference Scheffer, Berkovic, Capovilla, Connolly, French, Guilhoto and Zuberi2017). Patients with monogenic or genetic syndromes or with not well-defined etiology were excluded.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Clinical and Neurological Characteristics
We collected the following demographic data of the sample: gender, handedness, age at diagnosis, and comorbidities.
Patients underwent diagnostic procedures to define the type of epilepsy, etiology, and, when possible, epileptic syndrome, according to the 2017 Position Paper of ILAE Commission for Classification and Terminology (Scheffer et al., Reference Scheffer, Berkovic, Capovilla, Connolly, French, Guilhoto and Zuberi2017).
The diagnostic process included clinical-anamnestic and neurological evaluations, Video-EEG during wakefulness and sleep, long-term EEG monitoring (in cases where routine EEG was not sufficient for classification), high-resolution cerebral MRI, and, when indicated, 18 F-FDG PET-MRI.
Focal epilepsies were defined as structural when clinical or electro-clinical semiology of the seizures was attributable to the structural alterations which were shown on MRI. In structural epilepsies, we also included cases in which, although there was no precise etiological diagnosis (negative MRI or aspecific findings), the electro-clinical data, the monomorphism of the seizures during evolution, the pharmacoresistance/pharmaco-dependence and, if available, PET-MRI data, oriented toward a structural etiology.
The definition of a specific syndrome is possible only considering the presence of a cluster of features incorporating seizure types, EEG data, neuroimaging, and comorbidities.
Data on seizure status and medication at each evaluation were collected and considered in the analysis. Seizure frequency was evaluated in the 3 months preceding neuropsychological assessment. It was defined as: daily (at least one seizure/day), weekly (at least one seizure/week), monthly (more than one seizure/month), sporadic (one seizure/month or less), as well as seizure-free patients. Medication has scored as follows: no medication, monotherapy, and politherapy. The presence of comorbidities has been evaluated and summarized as follows: no comorbidities, psychopathological comorbidities, and medical comorbidities.
Neuropsychological Assessment
For each evaluation, we collected the age of the child and the time elapsed from the diagnosis of epilepsy (expressed in months and defined as “illness duration”). The clinical setting in which neuropsychological assessments were performed is described in the following paragraph.
Children with a new diagnosis of epilepsy are sent to the neuropsychological service for an assessment. This evaluation is not always performed very close to the onset of epilepsy because ours is a tertiary referral hospital, and the diagnosis is often made in other centers. Furthermore, we want to avoid possible interfering effects associated with the diagnostic process. Subsequent evaluations are performed at least a year apart to avoid practice effects. Usually, children with a new onset of epilepsy underwent an initial evaluation and a follow-up; however, if there is a clinical indication, patients may perform multiple evaluations. The clinical indication may refer to trouble not only on cognitive function or academic problems but also on medical or factors associated with epilepsy without apparently cognitive counterpart. A structured neuropsychological evaluation characterizes each assessment carried out by a trained child neuropsychologist (E.C.). The following cognitive domains were assessed: abstract reasoning, using the Raven Colored Matrices (Raven, Raven, & Court, Reference Raven, Raven and Court1998); language, using the naming test and the semantic verbal fluency test (Bisiacchi, Cendron, Gugliotta, Tressoldi, & Vio, Reference Bisiacchi, Cendron, Gugliotta, Tressoldi and Vio2005); memory, using the digit span test and the Corsi block-tapping test, which evaluate short-term verbal and visual-spatial memory, the words list and list recall, which evaluate learning and long-term verbal memory (Bisiacchi et al., Reference Bisiacchi, Cendron, Gugliotta, Tressoldi and Vio2005), and the backward digit span test, which evaluates working memory (Bisiacchi et al., Reference Bisiacchi, Cendron, Gugliotta, Tressoldi and Vio2005); attention, using the Bells test (Stoppa & Biancardi, Reference Stoppa and Biancardi1997), which evaluates selective and sustained attention, and the Trial Making Test A (TMT A) (Scarpa et al., Reference Scarpa, Piazzini, Pesenti, Brovedani, Toraldo, Turner and Bottini2006), which evaluates scan and search speed; executive functions, using the phonemic verbal fluency test, which evaluates the ability to access the lexicon through a phonemic cue by setting up an adequate verbal search strategy (Bisiacchi et al., Reference Bisiacchi, Cendron, Gugliotta, Tressoldi and Vio2005); the Frontal Assessment Battery (FAB) (Scarpa et al., Reference Scarpa, Piazzini, Pesenti, Brovedani, Toraldo, Turner and Bottini2006), which evaluates frontal lobes functions; the Trial Making Test B (TMT B), which evaluates attention shifting (Scarpa et al., Reference Scarpa, Piazzini, Pesenti, Brovedani, Toraldo, Turner and Bottini2006); and visual-motor abilities, using the Rey–Osterrieth Complex Figure Test (Caffarra, Vezzadini, Dieci, Zonato, & Venneri, Reference Caffarra, Vezzadini, Dieci, Zonato and Venneri2002), which evaluates praxis and planning abilities.
Description, procedure, and references for all neuropsychological tasks used are reported in the Supplement Table A.
Statistical Analysis
Scores for the neuropsychological instruments were age-corrected and converted into z-scores, equivalent or standard scores, as appropriate, using published normative data. The z-scores indicate the deviation from the mean population score, which is set to 0, standard deviation 1. A z score of −2 (or less) comprises 2.5 % of the normal distribution and is considered to be significantly lower than average. Equivalent scores are a 5-point scale standardized after adjustment for age and education. An equivalent score of 0 is considered to be significantly lower than average, one a borderline score, and 2–5 average scores. Standard scores indicate the deviation from the mean population score, which is set to 10, standard deviation 3. A standard score of 4 (or less) is considered to be significantly lower than average.
We calculated a global index of dysfunctions for executive functions and attention from impairments of single functions: we classified this as a dysfunction if a patient obtained an impaired score on at least two tasks. This methodology provided dichotomous values, and it is useful in order to quantify a range of impairments in a unitary measure.
In order to test the linear effect of illness duration, the child’s age at each assessment was computed and used as a predictor (centered and considered as a fixed and random effect) in different multilevel models, in addition to the other target predictor (i.e., epilepsy category) and the control variable (i.e., the medication). Multilevel linear models were implied for phonemic fluency, TMT B, FAB, Bells accuracy, and rapidity. Multilevel logistic models were computed for attention and executive functioning global indexes. Since we were also interested in the moderation of the epilepsy category on the effect of illness duration, we tested two models for each dependent variable: one with the main effects and one with the main effects as well as the interaction effect of illness duration × diagnosis. Analyses were performed with R-software (R Core Team, 2020), using the lme4 package (Bates, Mächler, Bolker, & Walker, Reference Bates, Mächler, Bolker and Walker2015) for multilevel modeling, the Car package (Fox & Weisberg, Reference Fox and Weisberg2011) to obtain Type II Wald chi-square tests. R 2 calculation is based on Nakagawa, Johnson, & Schielzeth (Reference Nakagawa, Johnson and Schielzeth2017). In order to interpret the significant interaction effect of diagnosis and illness duration, the library effects (Fox, Reference Fox2003) were used to represent the results visually, and simple slope analysis was performed utilizing the reghelper package (Hughes, Reference Hughes2017). Finally, we repeated the main analyses controlling the models also for the potentially confounding effect of the seizure status, and results are reported in the supplementary materials.
RESULTS
The flow chart with the procedure of selection of the final sample is reported in Figure 1.
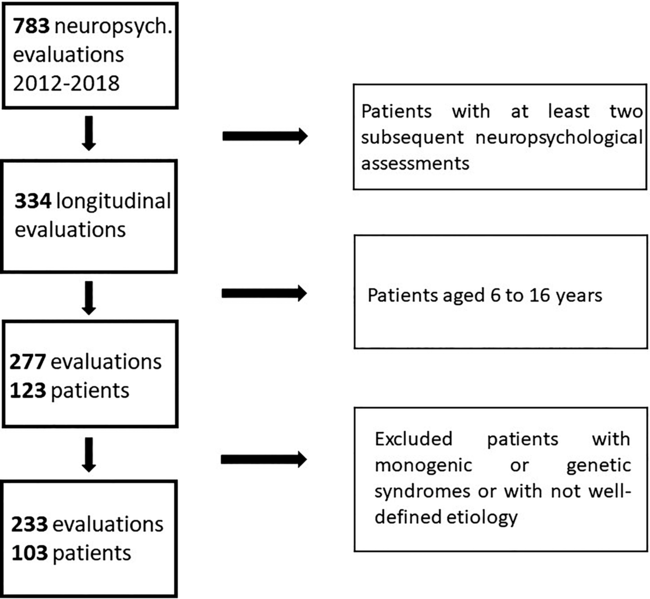
Fig. 1. Flow chart with the procedure of selection of the final sample.
According to the above-mentioned eligibility criteria, we identified 103 patients (focal self-limited N = 27; focal/structural N = 51; generalized N = 25) and 233 neuropsychological evaluations. Of the self-limited epilepsy group, 19 patients (70.4%) had two assessments, 6 (22.2%) three assessments, and 2 (7.4%) four assessments. Of the focal/structural epilepsy group, 40 patients (78.4%) had two assessments, 10 (19.6%) three assessments, and 1 (2%) had four assessments. Of the generalized epilepsy group, 21 patients (84%) had two assessments, 4 (16%) three assessments, and none had four assessments.
The percentage of medication used, alone or combined in a polytherapy, respectively, at first and at last assessment, is carbamazepine (21.9%, 18.2%), valproic acid (35.4%, 40.9%), oxcarbazepine (8.3%, 8%), levetiracetam (15.6%, 14.8%), ethosuximide (9.4%, 6.8%), methylphenidate (0%, 1.1%), lacosamide (1%, 2.3%), topiramate (1%, 0%), lamotrigine (4.2%, 5.7%), clobazam (1%, 2.3%), clonazepam (1%, 0%), and dintoin (1%, 0%).
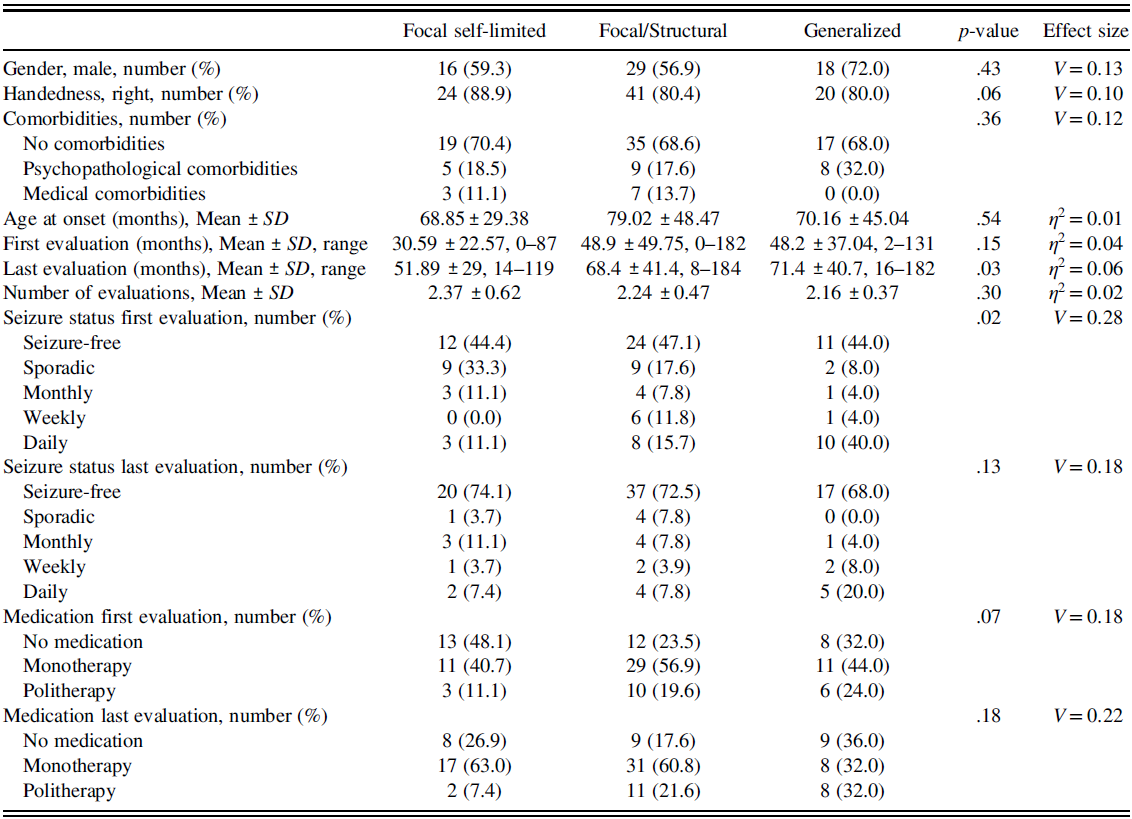
Table 1 reports the demographic data, comorbidities, temporal characteristics of the evaluations, seizure status, and medication at first and last evaluation considered for the three groups.
Table 1. For each examined epilepsy category, gender, handedness, comorbidities, age at onset, the time elapsed (in months) from the diagnosis to the first and last evaluation, the number of evaluations performed, and the age (in months) at the diagnosis, seizure status, and medication are reported
Within the focal self-limited epilepsy group, we were able to identify an epileptic syndrome in all the cases. Fourteen patients (51.8%) were classified as affected by childhood epilepsy with centrotemporal spikes, 9 (33.3%) by Panayiotopoulos syndrome, 3 (11.1%) by childhood occipital epilepsy and only one patient (3.7%) was classified as affected by Landau–Kleffner syndrome.
Within the generalized epilepsy group, we were able to define an epileptic syndrome in 21 cases (84%). Eight patients (32%) were classified as affected by childhood absence epilepsy, 5 (20%) by Epilepsy with myoclonic, atonic seizures, and 4 (16%) by epilepsy with eyelid myoclonias. Juvenile myoclonic epilepsy, juvenile absence epilepsy, epilepsy with generalized tonic-clonic seizures alone and myoclonic epilepsy in infancy were represented by one patient each (4%)
In order to further characterize the group of patients affected by focal epilepsy of structural etiology, we specify in Table 2 the different types of lesions that we have identified. However, in 15 cases (29.4%), the MRI was normal, and in 6 cases (11.7%), the neuroimaging data revealed only aspecific structural abnormalities (asymmetry of temporo-mesial structures, or in the organization of sulci and gyri). These patients, as explained in the methods, were classified as focal/structural epilepsies according to a comprehensive evaluation of electro-clinical phenotype, and PET data if available. Among the 30 patients with a defined structural lesion, in 20 cases (67%), it was unilateral (55% of lesions localized in the left hemisphere, 45% of lesions in the right hemisphere). In 10 patients (33%), the lesion involved both the cerebral hemispheres. In 17 patients (57%), the extension of the structural abnormality was limited to one lobe; frontal lobes were involved in 30.3% of cases, temporal lobes in 39.3% of cases, parietal lobes in 24.2% of cases and occipital lobes in 6% of cases.
Table 2. Different types of lesions of focal/structural epilepsies

Within the focal/structural epilepsy group, seven patients (13.7%) were surgically treated.
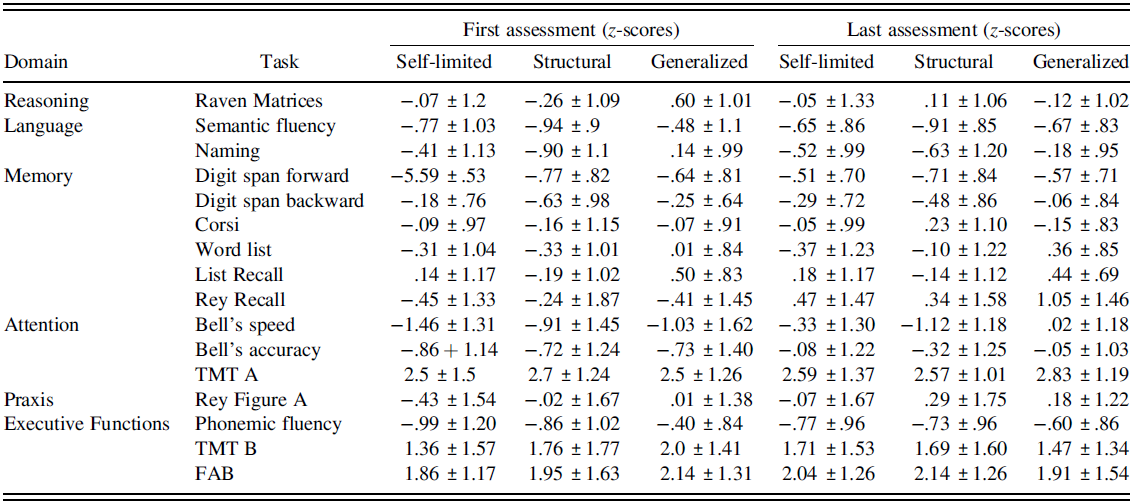
The percentage of deficits and mean z-scores reported in neuropsychological tasks at the first and last assessment for each group of patients are reported in Tables 3 and 4.
Table 3. Percentage of deficits (scores < 2 SD) obtained by the three group of patients at first and last assessment in neuropsychological tasks

Table 4. Mean z-scores (± standard deviations) obtained by the three groups of patients at first and last assessment in neuropsychological tasks
Results of the multilevel models aimed at testing the effects of illness duration, epilepsy category, and medication on executive tasks are presented in Table 5.
Table 5. Results of the regression analyses on the global executive index and the relative subscales

†p = .062; * p < .05; ** p < .01; *** p < .001.
The multilevel logistic regression found no change in time for the global executive index as well as no effect of the medication. Still, a trend toward statistical significance (p = .06) has been shown for the effect of the epilepsy category. No interaction between illness duration and epilepsy category was found. On the contrary, as shown in Figure 2, Phonemic fluency increases over time and its score significantly decreases when the medication increases. Also, the epilepsy categories differ in terms of Phonemic fluency, suggesting that focal self-limited epilepsies are associated with lower fluency, while focal/structural epilepsies obtain higher scores but lower when compared with generalized epilepsies. No significant interaction between illness duration and epilepsy category was found.

Fig. 2. Main effects of illness duration, epilepsy category, and medication on phonemic fluency.
Neither main effects nor interaction effects were found for TMT B, while a significant diagnosis × time interaction has been found for FAB. Figure 3 shows the effects, and subsequent simple slope analyses show that time has a positive non-significant effect on the epilepsy category = focal self-limited (b = .41, SE = .30, t = 1.38, p > .05, Cohen’s d = .75), a non-significant positive effect on epilepsy category = focal/structural (b = .04, SE = .15, t = .30, p > .05, Cohen’s d = .17), and a significant negative effect on epilepsy category = generalized (b = −.58, SE = .23, t = −2.40, p < .05, Cohen’s d = −1.02). In other words, only the patients with generalized epilepsy show a change of FAB over time, independently of the medication used.

Fig. 3. Interaction effect of illness duration and epilepsy category on FAB.
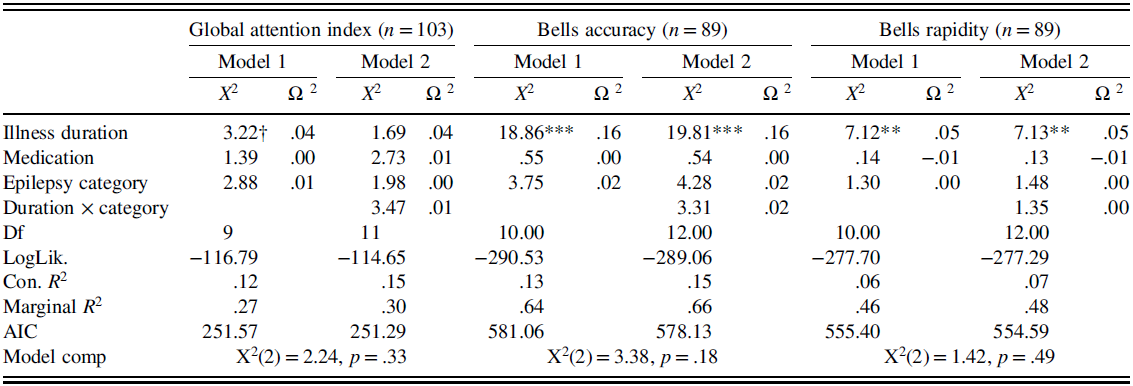
The results of the multilevel models aimed at testing the effects of illness duration, epilepsy category, and medication on attention are presented in Table 6.
Table 6. Results of the regression analyses on the global attention index and the relative subscales
† p = .070; * p < .05; ** p < .01; *** p < .001.
Regarding the global attention index, the multilevel logistic model shows no significant effect of medication and epilepsy category. Still, a trend toward statistical significance (p = .07) suggests that attention tends to increase over time. No interaction between illness duration and epilepsy category was found. Bells accuracy and rapidity are significantly increased over time, but neither main effects of medication of type of epilepsy nor interaction effects were found.
The main analyses were repeated, controlling for the potentially confounding effect of seizure status. Results are presented in Supplement tables (Tables B and C) and show that the associations remain unchanged in terms of effect sizes and statistical significance.
DISCUSSION
In the present study, we found a high percentage of executive and attention deficits in all three categories of examined patients, namely focal/structural epilepsies, so-called focal self-limited, and idiopathic generalized epilepsies.
Interestingly, by considering executive dysfunctions, patients with focal/structural epilepsies were the most affected independently of localization of the epileptogenic zone (the group contains several different localizations) and the deficit appeared to be persistent over time. By contrast, we did not find significant differences in frequency of attention impairments between the three groups of patients and an ameliorative effect of illness duration was documented in all the patients: at the last evaluation, attention abilities appeared to get better, independently of the epilepsy category.
Existing Literature
The finding of a high percentage of executive deficits in our cohort is consistent with the previous literature (Kavanaugh, Scarborough, & Salorio, Reference Kavanaugh, Scarborough and Salorio2015; Schraegle & Titus, Reference Schraegle and Titus2016). Impairments have been reported among a broad variety of seizure types and etiologies (Parrish et al., Reference Parrish, Geary, Jones, Seth, Hermann and Seidenberg2007; Hermann, Seidenberg, & Jones, Reference Hermann, Seidenberg and Jones2008) and have been documented even at the onset of epilepsy (Filippini et al., Reference Filippini, Ardu, Stefanelli, Boni, Gobbi and Benso2016; Parrish et al., Reference Parrish, Geary, Jones, Seth, Hermann and Seidenberg2007). However, empirical studies that directly compare cognitive functioning in children with different epileptic syndromes are very scarce. The majority of studies focused on risk factors, such as medication or seizure frequency, and often based on a retrospective evaluation of nationwide registries. However, some remarkable works have been conducted cross-syndrome comparisons. Fastenau et al. (Reference Fastenau, Johnson, Perkins, Byars, DeGrauw, Austin and Dunn2009) compared several types of epilepsy, both generalized and “localization-related,” with controls sibling. They found that children with symptomatic/cryptogenic etiology performed worse than those with idiopathic etiology on verbal memory and learning. For attention/executive/construction, both localization-related cryptogenic and generalized idiopathic absence groups scored lower than siblings. Another remarkable study is that of Hermann et al. (Reference Hermann, Seidenberg and Jones2008), who searched for cognitive phenotypes in children with new-onset focal and generalized idiopathic epilepsies. Confirmatory factor analysis identified five cognitive factors (verbal, perceptual, speed, attention, and executive), and latent class analysis identified three clusters of participants with epilepsy: (1) average and similar to controls, (2) mild impairment across multiple cognitive domains, and (3) impairment across all domains with severe attentional impairment. A subsequent effort of the same group investigated the neuropsychological profile of children who have new-/recent-onset idiopathic generalized epilepsy and idiopathic localization-related (Jackson et al., Reference Jackson, Dabbs, Walker, Jones, Hsu, Stafstrom and Hermann2013). They found considerable cognitive abnormality at baseline, including patterns of shared abnormalities across syndromes (e.g., psychomotor slowing) as well as unique syndrome-specific cognitive effects (e.g., executive function in idiopathic generalized epilepsy and language/verbal memory in idiopathic localization-related epilepsy). Academic difficulties are evident in approximately 50% of the children with epilepsy, affecting all syndrome groups to an equal degree. Lopes and colleagues examined the IQ (Lopes et al., Reference Lopes, Simões, Monteiro, Fonseca, Martins, Ventosa and Robalo2013) and memory abilities (Lopes, Monteiro, Fonseca, Robalo, & Simões, Reference Lopes, Monteiro, Fonseca, Robalo and Simões2014) in three common epilepsy syndromes (frontal lobe epilepsy, childhood absence epilepsy, and benign epilepsy with centrotemporal spikes). They showed that frontal lobe epilepsy has lower intelligence and memory scores. Also, they showed that type of epilepsy and duration of epilepsy were the best indicators of intellectual functioning and memory problems. Literature reported other interesting examples of syndromes comparisons (Cheng et al., Reference Cheng, Yan, Gao, Xu, Zhou and Chen2017; Culhane-Shelburne et al., Reference Culhane-Shelburne, Chapieski, Hiscock and Glaze2002; Law, Smith, & Widjaja, Reference Law, Smith and Widjaja2018; Nolan et al., Reference Nolan, Redoblado, Lah, Sabaz, Lawson, Cunningham and Bye2004; Riccio, Pliego, Cohen, & Park, Reference Riccio, Pliego, Cohen and Park2015).
Discussion of Our Results on Executive Functions
Although executive problems were overrepresented in each group, our study showed that executive functions were strongly and pervasively affected in focal/structural epilepsies. This result is particularly interesting if we consider that this group comprises patients with different structural abnormalities and localizations of the seizure focus. Executive dysfunctions were originally described on the basis of patterns of deficits observed in patients with frontal lobe lesions (Stuss & Benson, Reference Stuss and Benson1984; Stuss, Reference Stuss2011; Rabinovici, Stephens, & Possin, Reference Rabinovici, Stephens and Possin2015). Our observations are coherent with recent acquisitions, according to whose executive function depends on distributed neural networks that not only involve the prefrontal cortex prominently but also include parietal cortex, basal ganglia, thalamus, cerebellum, and white matter (Cainelli, Mioni, Boniver, Bisiacchi, & Vecchi, Reference Cainelli, Mioni, Boniver, Bisiacchi and Vecchi2019; Cainelli, Arrigoni, & Vedovelli, Reference Cainelli, Arrigoni and Vedovelli2020; Collette et al., Reference Collette, Van Der Linden, Laureys, Delfiore, Degueldre, Luxen and Salmon2005; Monchi et al., Reference Monchi, Petrides, Strafella, Worsley and Doyon2006; Rabinovici et al., Reference Rabinovici, Stephens and Possin2015). It has also been shown in other pathological conditions (Cainelli, Nosadini, Sartori, & Suppiej, Reference Cainelli, Nosadini, Sartori and Suppiej2019). Even if specific effects on executive function may be more likely with seizures originating from the frontal lobe, focal seizures arising from other lobes may result in secondary impairments (Guimarães et al., Reference Guimarães, Li, Rzezak, Fuentes, Franzon, Augusta Montenegro and Guerreiro2007; Rzezak et al., Reference Rzezak, Fuentes, Guimarães, Thome-Souza, Kuczynski, Li and Valente2007), determining interferences in the distributed neural networks underlying executive functions (Pereira et al., Reference Pereira, Alessio, Sercheli, Pedro, Bilevicius, Rondina and Cendes2010; Tracy et al., Reference Tracy, Osipowicz, Spechler, Sharan, Skidmore, Doucet and Sperling2014; Vlooswijk et al., Reference Vlooswijk, Vaessen, Jansen, De Krom, Majoie, Hofman and Backes2011). Interestingly, numerous functional imaging studies in patients with temporal lobe epilepsy have demonstrated altered connectivity in the default mode networks as a probable consequence of disrupted networks in the frontal lobe (Liao et al., Reference Liao, Zhang, Pan, Mantini, Ding, Duan and Chen2010; Lin, Riley, Juranek, & Cramer, Reference Lin, Riley, Juranek and Cramer2008; Riederer et al., Reference Riederer, Lanzenberger, Kaya, Prayer, Serles and Baumgartner2008; Vlooswijk et al., Reference Vlooswijk, Vaessen, Jansen, De Krom, Majoie, Hofman and Backes2011; Waites et al., Reference Waites, Briellmann, Saling, Abbott and Jackson2006).
In our patients with focal/structural epilepsies, executive function impairments were more frequent than in patients affected by focal and generalized epilepsies without structural damage. This suggests that the presence of a structural alteration may determine a more pervasive and persistent disruption of diffuse cerebral networks compared to other types of non-lesional epilepsies, including the so-called system epilepsies (Avanzini et al., Reference Avanzini, Manganotti, Meletti, Moshé, Panzica, Wolf and Capovilla2012). Interestingly, Rzezak and colleagues already showed that symptomatic epilepsy might determine worse executive performances compared to the so-called cryptogenic epilepsy (Rzezak et al., Reference Rzezak, Fuentes, Guimarães, Thome-Souza, Kuczynski, Li and Valente2007).
Discussion of Our Results on Attention
Attention involves several different components and partially overlap with executive functions; previous studies showed that children with epilepsy exhibit a wide range of attention difficulties (D’Alessandro et al., Reference D’Alessandro, Piccirilli, Tiacci, Ibba, Maiotti, Sciarma and Testa1990; Gascoigne et al., Reference Gascoigne, Smith, Barton, Webster, Gill and Lah2017; Semrud-Clikeman & Wical, Reference Semrud-Clikeman and Wical1999) and a high percentage of inattentive-type ADHD (Dunn & Kronenberger, Reference Dunn and Kronenberger2005; Gascoigne et al., Reference Gascoigne, Smith, Barton, Webster, Gill and Lah2017). However, a cross-sectional design (e.g., Cnaan et al., Reference Cnaan, Shinnar, Arya, Adamson, Clark, Dlugos, Hirtz, Masur and Glauser2017; D’Alessandro et al., Reference D’Alessandro, Piccirilli, Tiacci, Ibba, Maiotti, Sciarma and Testa1990; Deonna et al., Reference Deonna, Zesiger, Davidoff, Maeder, Mayor and Roulet2000; Fonseca Wald et al., Reference Fonseca Wald, Klinkenberg, Voncken, Ebus, Aldenkamp, Vles and Debeij-Van Hall2019; Masur et al., Reference Masur, Shinnar, Cnaan, Shinnar, Clark, Wang and Glauser2013; Shinnar et al., Reference Shinnar, Shinnar, Cnaan, Clark, Dlugos, Hirtz and Glauser2017), short follow-up periods and a lack of proper age bias correction (Cnaan et al., Reference Cnaan, Shinnar, Arya, Adamson, Clark, Dlugos, Hirtz, Masur and Glauser2017; Glauser et al., Reference Glauser, Cnaan, Shinnar, Hirtz, Dlugos, Masur and Adamson2010; Masur et al., Reference Masur, Shinnar, Cnaan, Shinnar, Clark, Wang and Glauser2013; Shinnar et al., Reference Shinnar, Shinnar, Cnaan, Clark, Dlugos, Hirtz and Glauser2017) are significant limitations of these studies, making further confirmation necessary. In this regard, our paper based on well-defined eligibility criteria, comparable protocols, and an extended follow-up - confirmed this observation, showing a high percentage of attention deficits without significant differences in frequency between the three groups of examined patients in our cohort. Moreover, an ameliorative effect of time was documented in all the patients independent of the epilepsy category after a consistent longitudinal follow-up, similar to what was previously observed by Fonseca Wald et al. (Reference Fonseca Wald, Klinkenberg, Voncken, Ebus, Aldenkamp, Vles and Debeij-Van Hall2019) in children with absent epilepsy after a follow-up time of more than 12 months, in both children with and without seizure freedom.
The link between attention function and epilepsy is complex; performance in attention skills may be affected by different factors, mainly by neurologic factors, such as ictal and interictal epileptiform activity (Marston, Besag, Binnie, & Fowler, Reference Marston, Besag, Binnie and Fowler1993) and antiepileptic drugs (Loring & Meador, Reference Loring and Meador2004; Schmitz, Reference Schmitz2006). Interestingly, the favorable course of attention displayed in our work is mainly due to the fact that the trend has resulted in being independent of the antiepileptic treatment. Conversely, the potential link between other clinical, neurophysiologic, environmental, and psychological variables has not been explored in our work due to the size of the sample, representing a limitation in our study.
The evolution in time of the attention performance is intriguing and not easy to interpret. The phenomenon is a cross-disease characteristic and did not appear to associate with other clinical variables, such as medication and seizure status. Therefore, we are tempted to interpret it as a psychological factor. Attention is strongly associated with psychological functioning and, in particular to anxiety and depression. It is reasonable to suppose that psychological factors might worsen the attention performance close to diagnosis time. This influence could wane over time, contributing at least in part to the improvement course of attention disturbances (Eysenck, Derakshan, Santos, & Calvo, Reference Eysenck, Derakshan, Santos and Calvo2007; Grillon, Robinson, Mathur, & Ernst, Reference Grillon, Robinson, Mathur and Ernst2016). At a confirmation, it has shown that in children with new/recent-onset idiopathic generalized and with localization-related epilepsies, behavioral problems are present near the time of diagnosis and tend to abate over time (Zhao et al., Reference Zhao, Rathouz, Jones, Jackson, Hsu, Stafstrom and Hermann2015). Clinicians working with psychiatric patients (in particular with anxiety disorders and depression) refer to the concept of “cognitive bias,” a wide range of patterns of dysfunctions – such as attentional bias –due to psychological disturbances. The first evaluation in our Department is not usually performed very close to the onset of epilepsy in order to avoid the effects of the distress due to the new diagnosis. The stigma that, unfortunately, still characterizes this condition may cause patients and their families a long period of distress with a negative impact on the initial neuropsychological presentation. In particular, the parental depression and anxiety associated with these levels of stress could initiate a dysfunctional cycle where dysfunctional parental involvement worsens the already challenged developmental trajectory of the child (Cottrell & Khan, Reference Cottrell and Khan2005; De Carli, Riem Madelon, & Parolin, Reference De Carli, Riem Madelon and Parolin2017; Sacchi et al., Reference Sacchi, De Carli, Vieno, Piallini, Zoia and Simonelli2018).
Conclusion
In conclusion, our results confirmed the high percentage of deficits in executive abilities and attention in epileptic populations found in previous studies. Patients with focal/structural epilepsies were mostly affected in the executive domain, independent of localization of the seizure focus, and the deficits persisted over time. By contrast, we did not find significant differences between the three groups of patients in the attention domain and an ameliorative effect of illness duration was documented in all patients. Even if similar problems have already been found in previous literature, few studies in the literature compare different epilepsy syndromes; therefore, despite the several limitations of this study, we add new information about the role of etiology and the evolution of deficits in time. Furthermore, we suggested a possible influence of psychological factors, as shown in other social and academic problems. Executive functions and attention have been shown to play a pivotal role in supporting other neuropsychological functions that may be impaired among patients with pediatric epilepsy (Black et al., Reference Black, Shih, Sepeta, Facella-Ervolini, Isquith and Berl2019). They are strong predictors of adaptive functioning within this population (Culhane-Shelburne et al., Reference Culhane-Shelburne, Chapieski, Hiscock and Glaze2002). Weak executive skills and attention also predict psychiatric comorbidity and behavioral problems among children with epilepsy (Alfstad et al., Reference Alfstad, Torgersen, Van Roy, Hessen, Hansen, Henning and Lossius2016; Baum et al., Reference Baum, Byars, deGrauw, Dunn, Bates, Howe and Austin2010) and have consistently been associated with decreased quality of life among pediatric patients (Love et al., Reference Love, Webbe, Kim, Lee, Westerveld and Salinas2016; Schraegle & Titus, Reference Schraegle and Titus2016; Sherman, Slick, & Eyrl, Reference Sherman, Slick and Eyrl2006). Interestingly, it has shown that training based on executive functions may improve the quality of life of children with epilepsy (Schraegle & Titus, Reference Schraegle and Titus2016). Thus, they remain an essential target for evaluation and the implementation of prompt rehabilitative intervention. Future research might explore the efficacy of an integrative intervention program, which takes care of both the cognitive and the psychological aspects, and their synergistic action.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
CONFLICT OF INTEREST
The authors have nothing to disclose.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617720001125











