INTRODUCTION
Body representation (BR) is a complex process involving various sources of top-down and bottom-up information (e.g., visual, proprioceptive, interoceptive) that is continuously and constantly updated (de Vignemont, Reference de Vignemont2011; Karnath & Baier, Reference Karnath and Baier2010; Palermo, Di Vita, Piccardi, Traballesi, & Guariglia, Reference Palermo, Di Vita, Piccardi, Traballesi and Guariglia2014). It is not a homogeneous concept, and several theoretical models have been proposed (Sirigu, Grafman, Bressler, & Sunderland, Reference Sirigu, Grafman, Bressler and Sunderland1991; Berlucchi & Aglioti, Reference Berlucchi and Aglioti2010; Corradi-Dell’Acqua & Rumiati, Reference Corradi-Dell’Acqua and Rumiati2007). The two main taxonomies are the dyadic taxonomy (Paillard, Reference Paillard1980), which distinguishes between body schema and body image, and the triadic taxonomy (Schwoebel & Coslett, Reference Schwoebel and Coslett2005), which maintains the proposed subdivision although it splits further the body image notion into two components, namely topological map of the body and body semantics. Neuropsychological evidence of multiple dissociations among different BRs supports the triadic taxonomy, suggesting that three different types of BR contribute to body knowledge, as it follows: (i) the body schema, which refers to the dynamic representation of body parts and their relative positions and contributes to the performance of movements by providing information about the dynamic position of each body part in relation to other parts (Schwoebel, Boronat, & Coslett, Reference Schwoebel, Boronat and Coslett2002; Schwoebel & Coslett, Reference Schwoebel and Coslett2005); (ii) the body structural representation (also called the visuospatial body map), which refers to a topological map of the body and contains data (mainly visual) about the location of body parts, the borders of the body, and distance relations between body parts (Buxbaum & Coslett, Reference Buxbaum and Branch Coslett2001; Schwoebel & Coslett, Reference Schwoebel and Coslett2005); (iii) the body semantics, which refers to a lexical-semantic representation of the body and contains the names of body parts and their functions, relations, and associations with objects (Coslett, Saffran, & Schwoebel, Reference Coslett, Saffran and Schwoebel2002; Schwoebel & Coslett, Reference Schwoebel and Coslett2005). Deficit of BRs has been analyzed mainly in single case reports or small group studies (i.e., Buxbaum & Coslett, Reference Buxbaum and Branch Coslett2001; Guariglia, Piccardi, Allegra, & Traballesi, Reference Guariglia, Piccardi, Allegra and Traballesi2002; Laiacona, Allamano, Lorenzi, & Capitani, Reference Laiacona, Allamano, Lorenzi and Capitani2006; Palermo et al., Reference Palermo, Di Vita, Piccardi, Traballesi and Guariglia2014; Di Vita et al., Reference Di Vita, Palermo, Piccardi, Di Tella, Propato and Guariglia2017), showing that the impairment of a specific BR would be associated with a specific neuropsychological deficit. For example, the disruption of the body schema could result in ideomotor apraxia (Buxbaum, Giovannetti, & Libon, Reference Buxbaum, Giovannetti and Libon2000), the disruption of the body structural representation could result in autotopoagnosia (Sirigu et al., Reference Sirigu, Grafman, Bressler and Sunderland1991) or personal neglect (Palermo et al., Reference Palermo, Di Vita, Piccardi, Traballesi and Guariglia2014; Di Vita et al., Reference Di Vita, Palermo, Piccardi, Di Tella, Propato and Guariglia2017; Committeri, Piervincenzi, & Pizzamiglio, Reference Committeri, Piervincenzi and Pizzamiglio2018), and the disruption of body semantics could contribute to body-specific aphasia (Suzuki, Yamadori, & Fuji, Reference Suzuki, Yamadori and Fuji1997). Systematic group studies on different BRs in patients with unilateral brain damage are still rare, and to the best of our knowledge, only two studies have been reported in literature (Razmus, Reference Razmus2017; Schwoebel & Coslett, Reference Schwoebel and Coslett2005). The seminal study by Schwoebel & Coslett (Reference Schwoebel and Coslett2005) systematically investigated different BRs in a large sample (n = 70) of unilateral brain-damaged patients, showing that at least one BR was affected in more than a half of patients with stroke (51% of patients). Body semantics and structural BR deficits were mainly associated with lesions lateralized to the left hemisphere, in particular with left temporal lesions, while no clear lateralization was observed for body schema deficits, mainly associated with parietal and dorsolateral frontal lesions. The study by Razmus (Reference Razmus2017), instead, was performed on a mixed sample of bilateral (70% of the sample) and unilateral brain-damaged patients, and deficits in at least one BR were found in 38 out of 47 patients (81% of patients). Overall these data suggest that BR deficits are widespread after stroke. However, in these two previous studies, representation deficits not directly related to body processing were not evaluated, leaving open the possibility that the reported BR deficits were not specific but due to more general cognitive deficits (e.g., deficit in language processing, in visuospatial and mental imagery skills). Indeed, Razmus (Reference Razmus2017) found associations between the performance in the BR tasks and deficits in working memory, visuospatial deficit, and naming of objects as evaluated with standard neuropsychological tests. Therefore, the purpose of the current study is to extend these findings conducting a systematic exploration of the impact of unilateral brain damage on BRs (i.e., body schema, body structural representation, and body semantics), using a specific battery of BR tests and comparing the performance of patients with right or left unilateral brain damage with that of a sample of healthy controls. Moreover, since a number of cognitive processes, beyond body processing, are necessary to perform BR tasks, we also administered similar control tasks, with no body-related stimuli, to disentangle the assessment of BRs from other cognitive functions.
Here we were interested in studying these three BRs and not to evaluate the clinical manifestations of BR alterations. In other words, an evaluation of how BR deficits can be related to several bodily disorders (e.g., autotopagnosia, body form agnosia, autoscopy, somatoparaphrenia, etc.; for a full list and description of these disorders, see de Vignemont, Reference de Vignemont2010), was out of the scope of the current study, and we refer the interested readers to previous works for specific studies on these disorders and clinical accounts (for studies on specific bodily disorders of neurological origins – such as autoscopy, somatoparaphrenia, anosognosia for hemiplegia, phantom limb, and personal neglect – see, e.g., Blanke & Mohr, Reference Blanke and Mohr2005; Vallar & Ronchi, Reference Vallar and Ronchi2009; Antoniello et al., Reference Antoniello, Kluger, Sahlein and Heilman2010; Gandola et al., Reference Gandola, Invernizzi, Sedda, Ferrè, Sterzi, Sberna, Paulesu and Bottini2012; Moro et al., Reference Moro, Pernigo, Tsakiris, Avesani, Edelstyn, Jenkinson and Fotopoulou2016; Committeri et al., Reference Committeri, Piervincenzi and Pizzamiglio2018; for studies on several body awareness disorders, see Herbet et al., Reference Herbet, Lemaitre, Moritz-Gasser, Cochereau and Duffau2019).
METHODS
Participants
Sixty-four patients with unilateral stroke and 41 healthy individuals (control participants, C: mean age = 58.39, SD = 7.03; mean education = 12.49, SD = 4.05) were enrolled in the study. Patients were divided into two main groups according to the side of lesion (22 with left brain damage, LBD; and 42 with right brain damage, RBD), and patients with RBD were, in turn, divided into two groups according to the presence of neglect (31 without neglect RBD-N; 11 right BD with neglect, RBD+N). Indeed RBD+N, compared with RBD-N, has shown to have distinctive clinical characteristics, such as deficit of awareness for visceral sensations (see Raimo et al., Reference Raimo, Boccia, Di Vita, Iona, Cropano, Ammendolia, Colao, Iocco, Angelillo, Guariglia, Grossi and Palermo2020), longer recovery, more functional disabilities (Paolucci et al., Reference Paolucci, Bureca, Multari, Nocentini and Matano2010), and distinct neural correlates (Committeri et al., Reference Committeri, Pitzalis, Galati, Patria, Pelle, Sabatini, Castriota-Scanderbeg, Piccardi, Guariglia and Pizzamiglio2007) that could affect BR performance. Age (χ 2 (3) = 4.43; p = .218) and education (χ 2 (3) = 6.92; p = .074) did not differ among groups. Even though there was a trend toward significance, which suggested that C had higher educational level compared with LBD (mean education = 9.91, SD = 4.51; p = .058) and RBD+N (mean education = 9.73, SD = 2.61; p = .055). This trend was not observed for the patient groups (i.e., the LDB, RBD+N, and RBD-N showed the same level of education). Demographic information and neuropsychological data for patients are shown in Table 1.
Table 1. Patients’ demographic and assessment data

Row scores are reported for the RCPM and after correction for age and education, all the scores were within the normal range.
LBD, left-brain-damaged patients; RPN, right-brain-damaged patients without neglect; RBD+N, right-brain-damaged patients with neglect; SA, subacute phase (10-90 days from stroke); Cr, cronic phase (>90 days from stroke); RMI, Rivermead Mobility Index; RCPM, Raven’s Progressive Matrices; N.A., Not available.
Patients were recruited from a population of in-patients at the IRCCS Fondazione Santa Lucia (Rome), the Rehabilitation Clinic, “Villa delle Magnolie” (Caserta), and “Aqua Salus” Rehabilitation Center (Catanzaro). Exclusion criteria for the patients were: the presence of more than one cerebrovascular accident, neoplastic or traumatic aetiology, cognitive deterioration, histories of psychiatric illness or substance abuse, and severe language comprehension deficits that prevented them from understanding the experimental tasks. At the time of the testing, none of the patients or caregivers spontaneously reported symptoms compatible with somatoparaphrenia, kinaesthetic hallucinations, supernumerary phantom limbs, anosognosia, or limb personification. Hemiplegia/hemiparesis was present in 76% of patients. Controls were recruited from the local community, and they did not show signs of neurological and/or psychiatric diseases or general cognitive impairment and history of substance abuse. The study was carried out in accordance with the Declaration of Helsinki, and ethical approval was obtained from the Ethics Committees of the Fondazione Santa Lucia IRCCS (Rome), of the University of Campania “Luigi Vanvitelli” (Caserta), and Calabria Region Ethical Committee, Catanzaro, Italy. Informed written consent was obtained from all the participants.
Neuropsychological Testing
Two trained neuropsychologists conducted an initial screening interview to exclude patients who showed severe language comprehension impairments. The Raven’s Coloured Progressive Matrices, (Raven, Reference Raven1938; Spinnler & Tognoni, Reference Spinnler and Tognoni1987) was given to patients to exclude the presence of deficit in abstract reasoning. Extrapersonal and personal neglect (see the supplementary material 1 for more details) were evaluated with the Standard Battery for the Evaluation of Hemineglect (Pizzamiglio, Judica, Razzano, & Zoccolotti, Reference Pizzamiglio, Judica, Razzano and Zoccolotti1989; Guariglia, Palermo, Piccardi, Iaria, & Incoccia, Reference Guariglia, Palermo, Piccardi, Iaria and Incoccia2013) and the Use of Common Objects test (Zoccolotti et al., Reference Zoccolotti, Antonucci and Judica1992). For a detailed description of the brain imaging data collection and results, see the supplementary material 2.
Body Representations Assessment
Currently there is a lack of normative tests probing the different BRs. For this reason, BR tasks were carefully choose among experimental tasks that have proved to be very sensitive to detect BR deficit in several previous studies on adults with brain damage (Canzano, Piccardi, Bureca, & Guariglia, Reference Canzano, Piccardi, Bureca and Guariglia2011; Guariglia & Antonucci, Reference Guariglia and Antonucci1992; Schwoebel & Coslett, Reference Schwoebel and Coslett2005) and children with cerebral palsy (Fontes et al., Reference Fontes, Cruz, Souto, Moura and Haase2017; Di Vita et al., Reference Di Vita, Cinelli, Raimo, Boccia, Buratin, Gentili, Inzitari, Iona, Iosa, Morelli, Ruggeri, Russo, Guariglia and Palermo2020). These tasks were also used in recent studies on the development of BRs in healthy children and young adults (Raimo et al., Reference Raimo, Iona, Di Vita, Boccia, Buratin, Ruggeri, Iosa, Guariglia, Grossi and Palermo2019) and on BR in older adults (Raimo et al., Reference Raimo, Boccia, Di Vita, Cropano, Guariglia, Grossi and Palermo2021).
Assessment of Body Semantics
Body semantics was evaluated using an Object-Body Part Association Task (adapted from Fontes, Moura, & Haase, Reference Fontes, Moura and Haase2014; Schwoebel & Coslett, Reference Schwoebel and Coslett2005; Raimo et al., Reference Raimo, Iona, Di Vita, Boccia, Buratin, Ruggeri, Iosa, Guariglia, Grossi and Palermo2019). The task included 20 stimuli in which participants had to correctly associate an object (e.g., hat) to the relative part of the body, choosing between two options (e.g., head and foot). In particular, an object (target) in the middle of the touchscreen and different body parts (response items) on the left and right sides of the touchscreen were shown. The participant had to associate the body part with the target object, by tapping one of the two response items. The task included 20 stimuli. Individual scores range from 0 to 20, with higher scores indicating better performance.
In the control task (i.e., a task involving the semantic processing of non-body-related stimuli), namely the Object-Room Association Task (Raimo et al., Reference Raimo, Iona, Di Vita, Boccia, Buratin, Ruggeri, Iosa, Guariglia, Grossi and Palermo2019), the participants had to correctly associate an object (e.g., armchair) to the relative room, choosing between two options (e.g., living room and bathroom). In particular, an object (target) in the middle of the touchscreen and two different rooms (response items) on the left and right sides of the touchscreen were shown. The participant had to associate the room with the target object, by tapping one of the two response items. The control task included 20 stimuli. Individual scores range from 0 to 20 with higher scores indicating better performance. Accuracy was recorded for both tasks, and the task presentation order was counterbalanced across participants. See Figure 1 for an example of the task items.
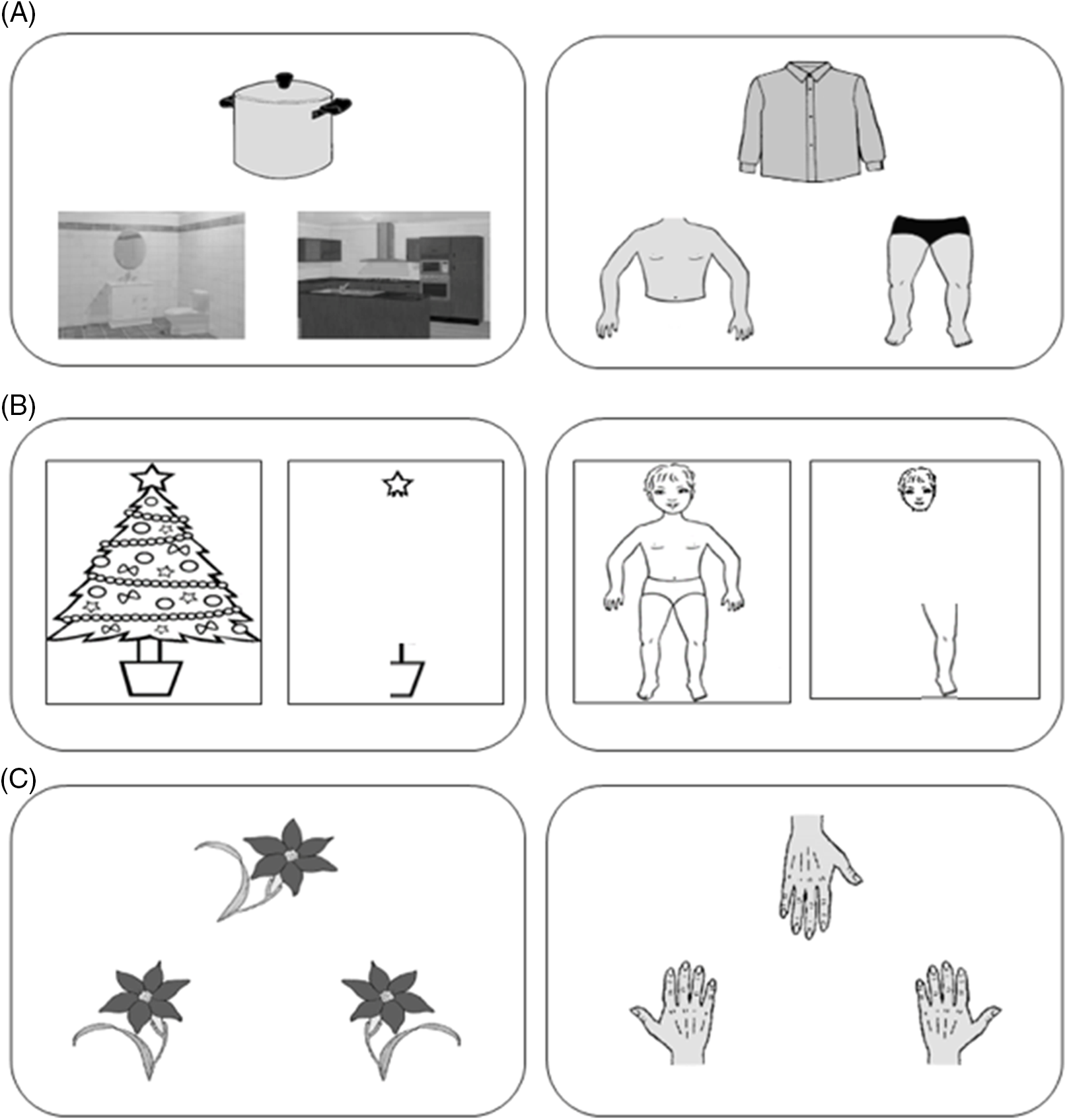
Fig. 1. Examples of items for the tasks involving body (right) and nonbody (left) processing. (A) An item of the task assessing the body semantics (Object-Body Part Association Task) is shown on the right panel, and an item of the control task (Object-Room Association Task) is shown on the left panel; (B) the task assessing the body structural representation (Frontal Body Evocation Task) is shown on the right panel and the control task (Christmas Three Task) on the left panel; (C) an item of the task assessing the body schema (Hand Laterality Task) is shown on the right panel, and an item of the control task (Object Laterality Task) is shown on the left panel.
Assessment of the Body Structural Representation
The body structural representation was evaluated using a computerized version of the Frontal Body Evocation Task (FBE) of the BR test (Daurat-Hmeljiak, Stambak, & Berges, Reference Daurat-Hmeljiak, Stambak and Berges1978; Raimo et al., Reference Raimo, Iona, Di Vita, Boccia, Buratin, Ruggeri, Iosa, Guariglia, Grossi and Palermo2019) that included a frame, on which the head of a child was depicted, and nine body parts (left and right legs, left and right hands, left and right arms, left and right parts of the chest and the neck). On a touch screen monitor, the participants were presented with one body part at a time, and their task was to place each part on the frame where only the head was depicted as reference part. In particular, a specific body part was shown on the right side of the frame and the participants had to place it at the correct position in the frame with respect to the reference of the head, by dragging it with their fingers. The position of the body part was recorded by the computer in terms of mm of distance from correct positioning; then, the body part disappeared and a new one was presented.
The control task (i.e., a task involving the visuospatial processing of non-body-related stimuli), namely the Christmas Tree Task (Raimo et al., Reference Raimo, Iona, Di Vita, Boccia, Buratin, Ruggeri, Iosa, Guariglia, Grossi and Palermo2019), included a frame, on which a star tree topper was depicted, and nine parts of a Christmas tree (left and right lower branches, left and right middle branches, left and right lower branches with trunks, left and right parts of jar, and the top). On a touch screen monitor, the participants were presented with one Christmas tree part at a time, and their task was to place each part on the screen frame where only the topper was depicted as reference part. In particular, a specific part of the tree was shown on the right side of the frame and the participants had to place it at the correct position in the frame with respect to the reference of the star tree topper, by dragging it with their fingers. The position of the part was recorded by the computer in terms of mm of distance from correct positioning; then, the Christmas tree part disappeared and a new one was presented. Accuracy was evaluated as the sum of mm of distance from the correct location of each body or tree parts (better performance was indicated by a few mm) for both tasks, and the task presentation order was counterbalanced across participants. See Figure 1 for an example of the task items.
Assessment of the Body Schema
Body schema was evaluated using a Hand Laterality Task (adapted and simplified from Parsons, Reference Parsons1987; Schwoebel & Coslett, Reference Schwoebel and Coslett2005; Raimo et al., Reference Raimo, Iona, Di Vita, Boccia, Buratin, Ruggeri, Iosa, Guariglia, Grossi and Palermo2019) in which participants were required to make a decision on the laterality of a single hand (20 stimuli, 10 left and 10 right stimuli) that could be presented at varying degrees of angular rotation (0, 45, 90, 270, 315 degrees) on a computer screen. In particular, a rotated hand (target), in the middle of the screen, and a left and a right hand (response items), respectively on the left and right lower part of the touchscreen, were shown. Participants had to decide whether the target hand was the left one or the right one, by mentally rotating the target hand and tapping one of the two response items. Individual scores range from 0 to 20 with higher scores indicating better performance.
The control task included a mental rotation task of nonbody stimuli, the Object Laterality Task (Raimo et al., Reference Raimo, Iona, Di Vita, Boccia, Buratin, Ruggeri, Iosa, Guariglia, Grossi and Palermo2019), where participants were required to make a decision on the laterality of a flower with a leaf positioned at the right or left base of the stem (20 stimuli, 10 left and 10 right stimuli) that could be presented at varying degrees of angular rotation (0, 45, 90, 270, 315 degrees) on the touch screen. In particular, a rotated flower (target), in the middle of the screen, and a flower with a leaf positioned at the left of the stem and a flower with a leaf positioned at the right of the stem (response items), respectively on the left and right lower part of the touch screen, were shown. Participants had to decide whether the flower target was the one with a leaf positioned at the left or the one with a leaf positioned at the right, by mentally rotating the flower target and tapping one of the two response items. Individual scores range from 0 to 20 with higher scores indicating better performance. To ensure that participants had fully understood the instructions, two practice trials were given for both tasks. Accuracy was recorded for both tasks, and the task presentation order was counterbalanced across participants. See Figure 1 for an example of the task items.
All the tasks were performed on a laptop (13.3” display) equipped with a touch screen monitor, and participants were seated on a chair in front of a desk with the laptop placed upon it. During testing, the participants were instructed to maintain the same position. No time limit was imposed, but they were solicited to respond immediately after presentation.
RESULTS
Single-Case Analyses
Crawford’s analyses, which were performed by using the computer program SINGLIMS_ES.exe (Crawford & Howell, Reference Crawford and Howell1998; Crawford & Garthwaite, Reference Crawford and Garthwaite2002), to determine whether the scores of individual patients were abnormal and significantly lower than those of the healthy control participants in the BR tasks without considering the performance on control tasks, are extensively reported in Supplementary material 3. Overall, these analyses revealed that deficits in at least one BR task were found in 64% of the patients (n = 41). See Figure 2.

Fig. 2. Side of brain lesion and body representation deficits percentage. LBD, left-brain-damaged patients; RBD, right-brain-damaged patients; RBD-N, right-brain-damaged patients without neglect, RBD+N, right-brain-damaged patients with neglect (i.e., extrapersonal neglect and/or personal neglect).
To identify patients with a selective deficit of BR (i.e., patients with a worse performance on BR tasks as compared with the respective control tasks) distinguishing between a pure deficit (i.e., a selective deficit in only one BR that is “pure” deficit of body semantics, “pure” deficit of body structural representation, and “pure” deficit of body schema) or mixed deficits of BR (i.e., a selective deficit in two or three body representations), we performed Crawford’s analysis using the computer program DISSOCS_ES.exe (Crawford, Garthwaite, & Porter, Reference Crawford, Garthwaite and Porter2010) that allows to detect significant dissociation in single cases, by comparing a single case’s difference in performance on a BR task and its respective control task with differences observed in the control group.
In eight patients, four LBD [Pt7: t(1,40) = 12.32, p < .0001; Pt16: t(1,40) = 1.78, p = .04; Pt18: t(1,40) = 1.78, p = .04; Pt19: t(1,40) = 1.78, p = .04] and four RBD-N [Pt41: t(1,40) = 10.59, p < .0001; Pt42: t(1,40) =1.78, p = .04; Pt43: t(1,40) = 1.78, p = .04; Pt48: t(1,40) =1.78, p = .04], the differences in performances between the Object-Body Part Association Task and the Object-Room Association Task were significantly different from the distribution of differences in controls, demonstrating the presence of a selective and pure impairment in body semantics.
In six patients, two LBD [Pt3: t(1,40) = 3.09, p = .001; Pt6: t(1,40) = 7.38, p < .0001] and four RBD-N [Pt24: t(1,40) = 3.62, p = .0004; Pt33: t(1,40) = 2.97, p = .002; Pt36: t(1,40) = 3.37, p = .008; Pt38: t(1,40) = 1.86, p = .03], the differences in performances between the FBE and the Christmas Tree Task were significantly different from the distribution of differences in controls, demonstrating the presence of a selective impairment in body structural representation.
In six patients, five LBD [Pt4: t(1,40) = 2.10, p = .02; Pt9: t(1,40) = 2.73, p = .004; Pt10: t(1,40) = 2.42, p = .01; Pt13: t(1,40) = 3.04, p = .002; Pt14: t(1,40) = 1.88, p = .03;] and one RBD-N [Pt51: t(1,40) = 1.98, p = .02], the differences in performances between the Hand Laterality Task and the Object Laterality Task were significantly different from the distribution of differences in controls, demonstrating the presence of a selective and pure impairment in body schema.
In three patients, one LBD [Pt5; association tasks: t(1,40) = 7.10, p < .0001; structural representation tasks: t(1,40) = 11.76, p < .001], one RBD-N [Pt39; association tasks: t(1,40) = 5.33, p < .0001; structural representation tasks: t(1,40) = 4.15, p < .001], and one RBD+N [Pt64; association tasks: t(1,40) = 5.33, p < .0001; structural representation tasks: t(1,40) = 4.15, p < .001], the differences in performances between the Object-Body Part Association Task and the Object-Room Association Task, and the differences between FBE and the Christmas Tree Task were significantly different from the distributions of differences in controls, demonstrating the presence of a mixed selective impairments in body semantics and body structural representation.
In two patients, one LBD [Pt11; structural representation tasks: t(1,40) = 2.17, p = .01; laterality tasks: t(1,40) = 2.10, p = .02;] and one RBD+N [Pt63; structural representation tasks: t(1,40) = 6.04, p < .0001; laterality tasks: t(1,40) =2.73, p = .004], the differences in performances between the FBE and the Christmas Tree Task and the differences between the Hand Laterality Task and the Object Laterality Task were significantly different from the distributions of differences in controls, demonstrating the presence of mixed selective impairments in body structural representation and body schema. See Supplementary material 3 for details on individual patients’ scores on BR and control tasks and on single-case analyses. Further details on the percentages of patients with a “selective” and “pure” BR deficit considering the patient groups (LBD, RBD-N, RBD+N) are reported in Tables 2 and 3 and showed in Figures 3 and 4.
Table 2. Percentage of patients with “selective” deficits in body representation tasks

LBD, left-brain-damaged patients; RBD, right-brain-damaged patients; RBD-N, right-brain-damaged patients without neglect, RBD+N, right-brain-damaged patients with neglect (i.e., extrapersonal neglect and/or personal neglect).
Table 3. Percentage of patients with a “pure” deficit in one of the three body representations

LBD, left-brain-damaged patients; RBD, right-brain-damaged patients; RBD-N, right-brain-damaged patients without neglect, RBD+N, right-brain-damaged patients with neglect (i.e., extrapersonal neglect and/or personal neglect).

Fig. 3. Side of brain lesion and “selective” body representation deficits percentage. LBD, left-brain-damaged patients; RBD, right-brain-damaged patients; RBD-N, right-brain-damaged patients without neglect, RBD+N, right-brain-damaged patients with neglect (i.e., extrapersonal neglect and/or personal neglect).
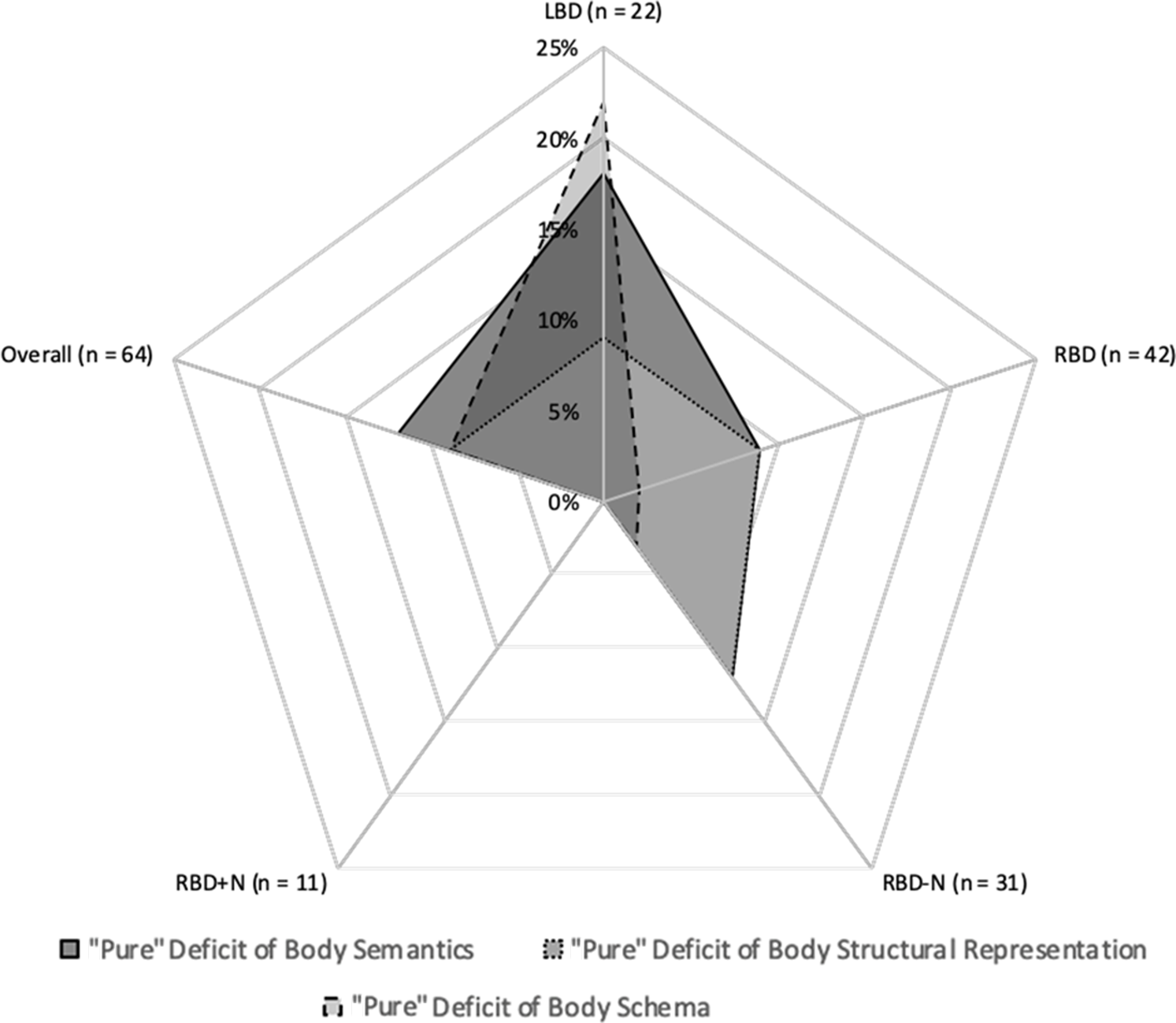
Fig. 4. Side of brain lesion and “pure” body representation deficits percentage. LBD, left-brain-damaged patients; RBD, right-brain-damaged patients; RBD-N, right-brain-damaged patients without neglect, RBD+N, right-brain-damaged patients with neglect (i.e., extrapersonal neglect and/or personal neglect).
Group Analyses
Group analyses were performed to evaluate the overall effect of unilateral brain damage on BRs and a possible hemispheric lateralization of BRs, disentangling the assessment of BRs from other cognitive skills required to perform the BR tasks. Kolmogorov–Smirnov test was applied to verify the normal distribution of data on accuracy scores; since the results showed that data were not normally distributed, nonparametric analyses were performed. In particular to disentangle the contribution of other cognitive skills involved in performing BR tasks and investigating the significant difference on BR tasks among groups, three Quade’s rank analyses of covariance were performed, following these steps: (i) we ranked the scores of each BR task (dependent variables: Object-Body Part Association Task; FBE; Hand Laterality Task) and of each control task (covariates: Object-Room Association Task; Christmas Tree Task; Object Laterality Task); (ii) we performed three linear regression analyses of the ranks of the dependent variable on the ranks of the covariate, saving the residuals. In particular, the first analysis performed a regression of the ranks of Object-Body Part Association Task on the ranks of the Object-Room Association Task (i.e., the respective control task), and body semantic residuals were calculated; the second analysis performed a regression of the ranks of the FBE on the ranks of the Christmas Tree Task (i.e., the respective control task), and body structural representation residuals were calculated; and the third regression analysis performed a regression of the ranks of the Hand Laterality Task on the ranks of the Object Laterality Task, and body schema residuals were calculated; (iii) to implement the Quade’s test computed by using IBM SPSS Statistics for Macintosh, Version 24.0, we performed three analyses of variance (ANOVA) with body semantics, or body structural representation, or body schema residuals as dependent variable, and the Group (LBD, RBD-N, RBD+N, C) as independent variable and years of education as covariate. The F test resulting from this ANOVA was the F statistic Quade’s test. Moreover, to analyse significant effects, Mann–Whitney U was performed and a Bonferroni correction for multiple comparisons was applied.
Concerning the body semantics (Object-Body Part Association Task), the rank analysis of covariance (Quade’s test) showed no significant main effect of Group (F (3, 103) = 1.57; p = .201) even when the years of education were added as covariate (F (3, 103) = 1.43; p = .239).
Concerning the body structural representation (FBE Task), the rank analysis of covariance showed a significant effect of Group (F (3, 103) = 5.18; p = .002) even when the years of education were added as covariate (F (3, 103) =5.14; p = .002). Post-hoc comparison showed that all patient groups performed significantly worse than controls (LBD vs. C: Mann–Whitney U = 187, p < .0001; RBD-N vs. C Mann–Whitney U = 283, p < .0001; RBD+N vs. C: Mann–Whitney U = 45, p < .0001) while the three patient groups showed a similar performance (LBD vs. RBD-N: Mann–Whitney U = 336, p = .928; LBD vs. RBD+N: Mann–Whitney U = 77, p= .093; RBD-N vs. RBD+N: Mann–Whitney U = 111, p = .089).
Concerning the body schema (Hand Laterality Task), the rank analysis of covariance (Quade’s test) showed a significant effect of the Group (F (3, 103) = 4.07, p = .009) even when the years of education were added as covariate (F (3, 103) = 3.43, p = .019). Post-hoc comparison showed that the LBD performed significantly worse than both C (Mann–Whitney U = 241, p = .002) and RBD-N (Mann–Whitney U = 228, p = .037), but no significant differences were found between LBD and RBD+N (Mann–Whitney U = 84, p = .166). Both the RBD groups performed similarly to controls (RBD-N vs. C: Mann–Whitney U = 528, p = .196; RBD+N vs. C: Mann–Whitney U = 176, p = .237) and to each other (RBD-N vs. RBD+N: Mann–Whitney U = 160, p = .767).
All the significant differences survived after Bonferroni correction for multiple comparisons, but the difference between LBD and RBD-N on the Hand Laterality Task (p = .037). Means and standard deviations of accuracy of performance on the BR for each group are shown in Table 4.
Table 4. Means and standard deviations on the body representation tasks in the four groups

C, control participants; LBD, left-brain-damaged patients; RBD-N, right-brain-damaged patients without neglect, RBD+N, right-brain-damaged patients with neglect (i.e., extrapersonal neglect and/or personal neglect).
DISCUSSION
The aim of the present study was to evaluate the three different BRs (the body schema, the body structural representation, and the body semantics) within the same sample of brain-damaged patients, disentangling the contribution of other cognitive skills involved in performing BR tasks and investigating the specific impact of left or right unilateral brain damage.
The incidence of disorders of BRs was very high in our sample when we considered the performance only in BR tasks (see Supplementary material 3 for details). Indeed, 64% of patients with unilateral brain damage were impaired, as compared with controls, on at least one BR task. This result is in line with the rate reported in the seminal study by Schwoebel and Coslett (Reference Schwoebel and Coslett2005), showing that 51% of patients with unilateral stroke were impaired on at least one BR measure. Thus current results, derived from a comparison with a very robust single case methodology and using a larger sample of healthy controls including both men and women (i.e., n = 41 vs. 18 healthy controls who were all women but one in Schowoebel & Coslett, 2005), further suggest that the body processing is often compromised after brain damage. Instead, Razmus (Reference Razmus2017) showed that 81% of patients were impaired on at least one BR measure. However, the higher incidence of BR deficits in the study by Razmus (Reference Razmus2017) could be related to the bilateral lesions that occurred in most cases while the present study and the one by Schwoebel’s and Coslett’s assessed only patients with unilateral brain damage.
An innovative aspect of the current study is the possibility to detect selective BR since, differently from these relevant previous studies (Schwoebel & Coslett, Reference Schwoebel and Coslett2005; Razmus, Reference Razmus2017), we controlled for the possible effect of cognitive skills required to perform the BR tasks (e.g., in visual processing, mental imagery, visuospatial attention, decision-making, etc.). The incidence of BR disorders was lower in our sample when performance on control tasks was considered: 37.5% of patients with unilateral brain damage were, indeed, impaired on at least one BR task, relative to controls, when compared with the respective control tasks (selective deficits). Moreover, we also identified pure deficits (i.e., a deficit in only one specific BR) in patients with unilateral brain damage finding that these were present in 31.2% of patients: 12.5% showed a selective deficit of the body semantics; 9.37% a selective deficit of the body structural representation; and 9.37% a selective deficit of the body schema. With respect to the hemispheric laterality, a selective and pure deficit in body schema was frequently detected in the sample of left-brain-damaged patients (i.e., selective deficit: 27.2% of the patients with LBD vs. 7.1% of the patients with RBD; pure deficit: 22.7% of the patients with LBD vs. 2.3% of the patients with RBD).
The presence of pure BR deficits is noteworthy since it underlines a certain degree of independence between these three BRs, at least in adulthood. In other words, although these three BRs can shape each other, above all during development (see Raimo et al., Reference Raimo, Iona, Di Vita, Boccia, Buratin, Ruggeri, Iosa, Guariglia, Grossi and Palermo2019; Aucliar & Jambaqué, Reference Auclair and Jambaqué2015), and there is evidence that their construction is based on their interactions (Pitron et al., Reference Pitron, Alsmith and de Vignemont2018), the selective pure deficits reported in this study further prove that they are functionally distinct.
Furthermore, we performed specific nonparametric statistical analyses (Quade’s rank analysis of covariance) that allowed us to evaluate a possible hemispheric lateralization and the overall effect of unilateral brain damage on BR processing, regardless other (and more general) cognitive processes required to perform BR tasks. In this case, the three patient groups (LBD, RBD-N, RBD+N) performed similarly to controls on the body semantics task (i.e., the Object-Body Part Association Task), whereas their performances were significantly worse than those of controls on the body structural representation (i.e., the FBE Task). Moreover, the LBD group showed performances significantly worse than those of controls and RBD-N on the body schema (i.e., Hand Laterality Task), although this latter comparison did not survive multiple comparison testing.
Current findings showed that the left hemisphere could play a pivotal role in the body schema in consistence with the previous neuropsychological literature finding that deficits in BR generally follow left hemispheric lesions (see, e.g., Semenza, Reference Semenza and Berndt2001). In particular, previous functional studies (Tomasino, Toraldo, & Rumiati, Reference Tomasino, Toraldo and Rumiati2003; Kosslyn, DiGirolamo, Thompson, & Alpert, Reference Kosslyn, DiGirolamo, Thompson and Alpert1998) assessing mental rotation of body (hand) and no-body (i.e., flag shapes, 3D cubes) stimuli showed that hand laterality judgments engendered activation in the left hemisphere, while laterality judgments of no body objects engendered activation in the right hemisphere, suggesting that only patients with left hemisphere lesions would be selectively impaired in mental rotation of body stimuli requiring motor strategy and body schema activation (Tomasino & Rumiati, Reference Tomasino and Rumiati2004). However, this result should be cautiously interpreted because the difference between LBD and RBD-N on Hand Laterality Task performances did not survive correction for multiple comparisons.
Current results suggest that the right and left hemispheres are both involved in the processing of the body structural representation, since this representation is specifically compromised in both left- and right-brain-damaged patients. The correct location of each body part has been suggested to involve the posterior parietal cortex and the intraparietal sulcus in the left side of the brain (Denes, Cappelletti, Zilli, Dalla Porta, & Gallana, Reference Denes, Cappelletti, Zilli, Dalla Porta and Gallana2000; Schwoebel, Coslett, & Buxbaum, Reference Schwoebel, Coslett and Buxbaum2001; Felician, Ceccaldi, Didic, Thinus-Blanc, & Poncet, Reference Felician, Ceccaldi, Didic, Thinus-Blanc and Poncet2003), so that damage to these regions may lead to autotopagnosia (Felician et al., Reference Felician, Romaiguère, Anton, Nazarian, Roth, Poncet and Roll2004). However, the body structural representation largely derived from visual input of others’ bodies through a self/other processing that has been suggested to have its neural underpinnings in frontoparietal cortex of the right side of the brain (Decety & Sommerville, Reference Decety and Sommerville2003; Vogeley & Fink, Reference Vogeley and Fink2003; Blanke & Arzy, Reference Blanke and Arzy2005). The role of the right hemisphere in processing spatial knowledge about the body is also strongly supported by previous studies on unilateral brain-damaged patients (Di Vita et al., Reference Di Vita, Palermo, Piccardi, Di Tella, Propato and Guariglia2017; Di Vita, Palermo, Boccia, & Guariglia, Reference Di Vita, Palermo, Boccia and Guariglia2019; Palermo et al., Reference Palermo, Di Vita, Piccardi, Traballesi and Guariglia2014) showing that deficit in tasks tapping on the body structural representation usually results from lesions within the right hemisphere, so much so that body structural representation deficit may be considered a clinical sign of personal neglect (Di Vita et al., Reference Di Vita, Palermo, Piccardi, Di Tella, Propato and Guariglia2017, Reference Di Vita, Palermo, Boccia and Guariglia2019). Our finding on the body structural representation allows to reconciliate previous data on stroke patients that suggested a prominent role of only one of the brain hemispheres (i.e., the right hemisphere in Di Vita et al., 2017; Reference Di Vita, Palermo, Boccia and Guariglia2019; Palermo et al., Reference Palermo, Di Vita, Piccardi, Traballesi and Guariglia2014; and the left hemisphere in Schwoebel & Coslett, Reference Schwoebel and Coslett2005) and to better discuss some issues that remained unresolved in previous literature, due to small sample sizes (e.g., Di Vita et al., Reference Di Vita, Palermo, Piccardi, Di Tella, Propato and Guariglia2017) or to failure to assess this BR taking into account possible deficits in other cognitive processes necessary to perform the task (e.g., Di Vita et al., Reference Di Vita, Palermo, Boccia and Guariglia2019; Palermo et al., Reference Palermo, Di Vita, Piccardi, Traballesi and Guariglia2014; Schwoebel & Coslett, Reference Schwoebel and Coslett2005).
In sum, our study confirms the hypothesis that BR deficits are a common consequence of left and right unilateral brain damage, excluding any doubt about the fact that these deficits are not due to more general impairments in cognitive functioning.
Considering the BR theoretical framework on the basis of the triadic taxonomy (Schwoebel & Coslett, Reference Schwoebel and Coslett2005; Sirigu et al., Reference Sirigu, Grafman, Bressler and Sunderland1991), our results show that selective and pure deficits in the conceptual and linguistic representation of the body (i.e., body semantics), in the sensory-motor representation of the body (i.e., body schema), and in the visuospatial map of the different body parts (i.e., body structural representation) were detected with the same frequency in this population of patients with brain damage. Furthermore, while a deficit of the body structural representation is a common consequence of lesions to both hemispheres, a deficit of the body schema would seem to be associated to the left brain damage. Therefore, current results shed precious light on how specific BR deficits, such as other cognitive dysfunctions (i.e., aphasia, apraxia, memory deficits), may be a consequence of unilateral brain damage and are not secondary to other more general cognitive deficits.
We used a battery of tasks specifically developed to assess the three BRs patterned upon the tasks used by Schwoebel and Coslett (Reference Schwoebel and Coslett2005) and in line with the previous literature (see Razmus, Reference Razmus2017; Di Vita et al., Reference Di Vita, Palermo, Boccia and Guariglia2019). Possible caveats are due to the use of only one task to assess each BR and to differences between the tasks developed to test different BR and relative scoring procedures (e.g., body schema and body semantics were assessed by means of choosing between two alternatives, whereas body structural representation was assessed by means of correct location of a stimulus in a frame measured in mm of distance). Therefore, further normative studies should be conducted to establish the different sensitivity of these tasks to assess each BR specifically. Moreover, we emphasize the need to evaluate BRs in clinical practice since they may be altered in various ways after central nervous system damage and result in a vast range of clinical manifestations (see de Vignemont, Reference de Vignemont2010). Further, BR deficits are often underreported due to the lack of a detailed exploration of a patient’s BR in routine neurological assessment (Antoniello et al., Reference Antoniello, Kluger, Sahlein and Heilman2010). BR deficits may affect motor outcomes, daily functioning, and quality of life of patients with unilateral brain damage, suggesting that these patients could benefit from specific neuropsychological rehabilitation training targets to treat BR disorders in association with standard rehabilitation training programs.
ACKNOWLEDGMENTS
We are thankful to Stefano Buratin and Francesco Ruggeri for their help with the development of the computerized version of the experimental tasks, to Stefano Buratin for his help with the development of the computerized version of the scoring sheets, to Professor Maurizio Iocco for allowing access to some patients, and to Maria Giovanna Caruso for helping with the recruitment of some patients.
FUNDING
This research has been supported by funding from Fondazione con il Sud to Liana Palermo (Brains2South – Project code: 2015-PDR-0248).
CONFLICT OF INTEREST
The authors have nothing to disclose.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617721000151










