Introduction
The management of large, unresectable pelvic tumours is a difficult clinical challenge. Patients with advanced-stage gynaecologic cancers often present with a high burden of symptoms and management in a fragile patient population can be very difficult. Large pelvic masses can cause significant gastrointestinal, genitourinary and pain symptoms. Leiomyosarcoma specifically is traditionally considered relatively radioresistant and has low-response rates to systemic chemotherapy with limited overall systemic options. Reference Naghavi, Yang and Latifi1 High radiation doses such as stereotactic body radiation therapy (SBRT) have been used in small lesions, but radiation treatment options for large tumours are limited by normal tissue dose constraints. Designing a treatment that delivers effective palliation and preserves the patient’s quality of life by minimizing potential side effects is challenging.
Spatially fractionated radiotherapy (SFRT), which intentionally delivers a very inhomogeneous radiation dose distribution to the tumour, is one promising approach for the treatment of large bulky tumours not amendable to surgery or high-dose stereotactic approaches. Reference Mohiuddin, Fujita and Regine2–Reference Snider, Molitoris and Shyu4 In SFRT, small parts of the tumour receive high SBRT-like doses, while the remainder of the tumour is treated at significantly lower doses. Reference Snider, Molitoris and Shyu4–Reference Wu, Perez and Zheng6 Since the whole target is not treated to a large dose, side effects caused by irradiation of normal tissue are usually mild. Reference Penagaricano, Moros and Ratanatharathorn7,Reference Mohiuddin, Stevens and Reiff8 SFRT is often followed up by a short course of conventional external beam radiation (EBRT) either immediately or after a short break as a split course treatment. GRID is an SFRT technique which does not require the use of intensity-modulated radiotherapy, unlike most SBRT plans. It avoids a lengthy treatment planning, QA and insurance authorization process and is well suited for the palliation of large, symptomatic lesions which often require urgent intervention. GRID can be delivered in several different ways, using single radiation field or a combination of intersecting fields and shaping the fields either with custom-designed blocks or a multileaf collimator (MLC). Reference Zhang, Wu and Zhang5,Reference Neuner, Mohiuddin and Vander Walde9
Case description
The patient is a 53-year-old female who presented with a large unresectable leiomyosarcoma of the uterus. The patient had advanced disease at diagnosis and progressed through initial chemotherapy (six cycles of Adriamycin, seven of docetaxel/gemcitabine). She underwent debulking surgery, but soon after developed a bulky pelvic recurrence. When her pain dramatically increased, she underwent imaging which noted increase in the size of her pelvic disease and hydronephrosis secondary to the mass causing obstruction of the ureter. She was also noted to have progression of metastatic disease with noted lung disease. At this juncture, radiation oncology was contacted for consideration of palliative pelvic radiation.
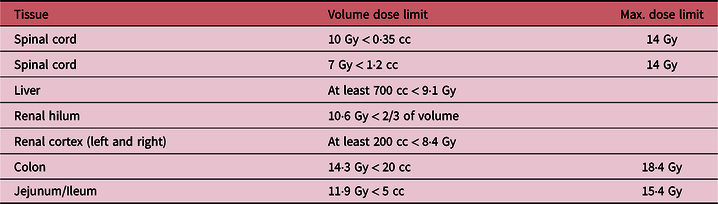
The clinical target volume (CTV) and the organs at risk (spinal cord, liver, kidneys, pancreas, bowels) were contoured on the treatment planning CT scan. Considering the size (945 cc) and extensive nature of the CTV, we opted for a crossfire GRID Reference Pokhrel, Bernard and Mallory10,Reference Pokhrel, Halfman and Sanford11 treatment plan. Based on the CTV, we created a 3D plan with 6 evenly spaced radiation beams (gantry angles of 30, 90, 150, 210, 270 and 330 degrees, all with collimator angle 90 degrees) with 6× or 10× energy. Each beam had a static MLC pattern consisting of 1 cm wide, alternating open and closed strips, the MLC opening constrained to the CTV. The resulting dose distribution consists of cylindrical high-dose areas in the sagittal and coronal planes, separated by areas of lower dose (see Figure 1). We prescribed 18 Gy to the isocenter. The dose-volume histogram is shown in Figure 2. The mean dose to the CTV was 11·3 Gy and 10%/50%/90% of the CTV received at least 16 .0/10·4/8·2 Gy. The minimum and maximum CTV doses were 3·5 Gy and 19·0 Gy. All single fraction SBRT normal tissue dose limits of American Association of Medical Physicists Task Group 101, Reference Benedict, Yenice and Followill12 shown in Table 1, were met. The treatment plan was verified by performing an independent monitor unit calculation and a dose measurement using an ArchCheck diode array (Sun Nuclear, Melbourne, FL).
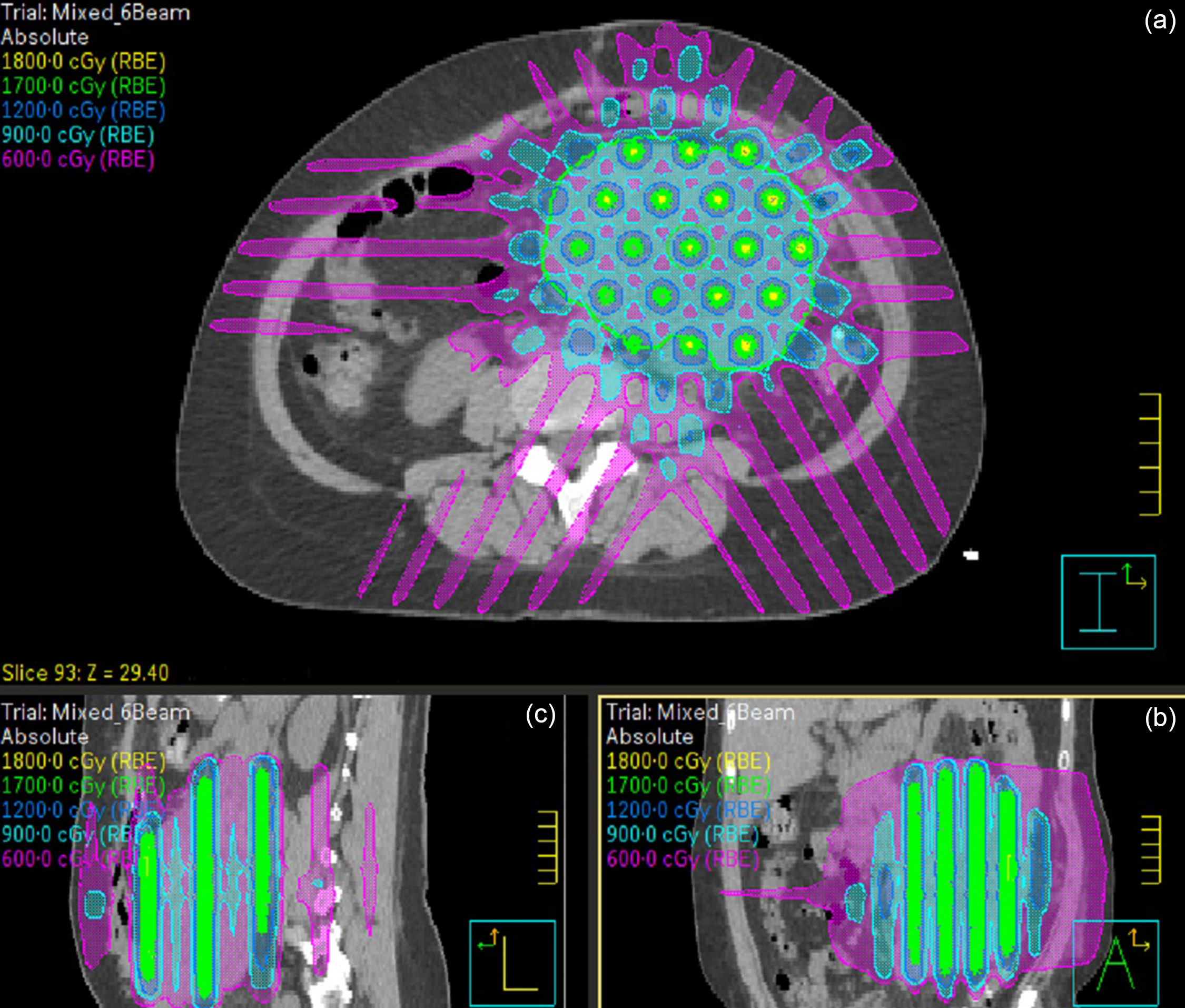
Figure 1. The isodose distribution in the axial (a; CTV contour in green), coronal (b) and sagittal (c) planes. The cylindrical GRID high-dose areas are formed perpendicular to the axial plane.
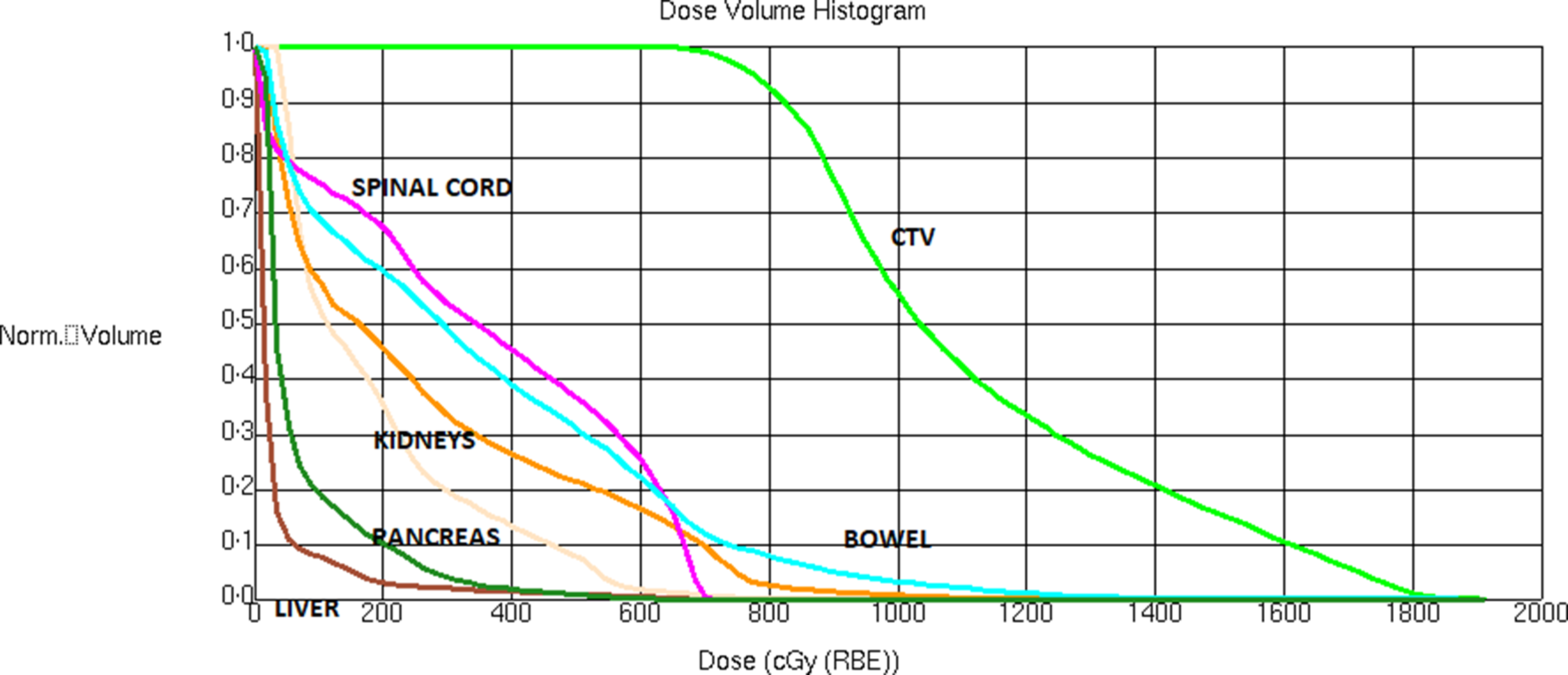
Figure 2. The dose-volume histogram of the GRID plan.
Table 1. The AAPM Task Group 101 single fraction dose limits for the organs relevant to this case (all limits were met by the plan).
The patient was positioned using a cone beam CT (CBCT) with a soft tissue match to the pelvic mass. The treatment time, from the beginning of the CBCT acquisition to the end of the last treatment field, was 12 min. Despite being in considerable pain, the patient was able to tolerate the treatment of this duration easily. Three weeks after the GRID treatment, a new treatment planning CT was acquired to complete a planned split course of palliative radiation. An EBRT plan, having two intensity-modulated arcs and a prescription dose of 4 Gy × 5 to an EBRT CTV of 524 cc, was created. The treatments were delivered four weeks after the initial GRID treatment.
The patient initiated additional systemic therapy (Pazopanib) after her course of radiation and was followed with surveillance CT imaging to assess response to therapy and to assess for metastatic progression. The primary tumour volume treated with SFRT decreased substantially from 945 cc at time of treatment to 276 cc at 5·5 months and further to 17 cc at 8·5 months (Figure 3). Her abdominal symptoms remained well controlled after radiation requiring no further oral pain medications. Based on the follow-up notes of physicians (1 month post-treatment until the death of the patient), the radiation therapy did not result in any acute toxicity. Her hydronephrosis was managed with stent placement when identified and secondary to stent placement and the decrease in tumour volume did not return. At 8·5 months post-treatment, a mass in the pancreatic tail, likely a new metastasis, was identified. At 9 months post-treatment, the patient died from progression of her metastatic disease in the lungs, but the primary tumour site had still not shown evidence of local progression.
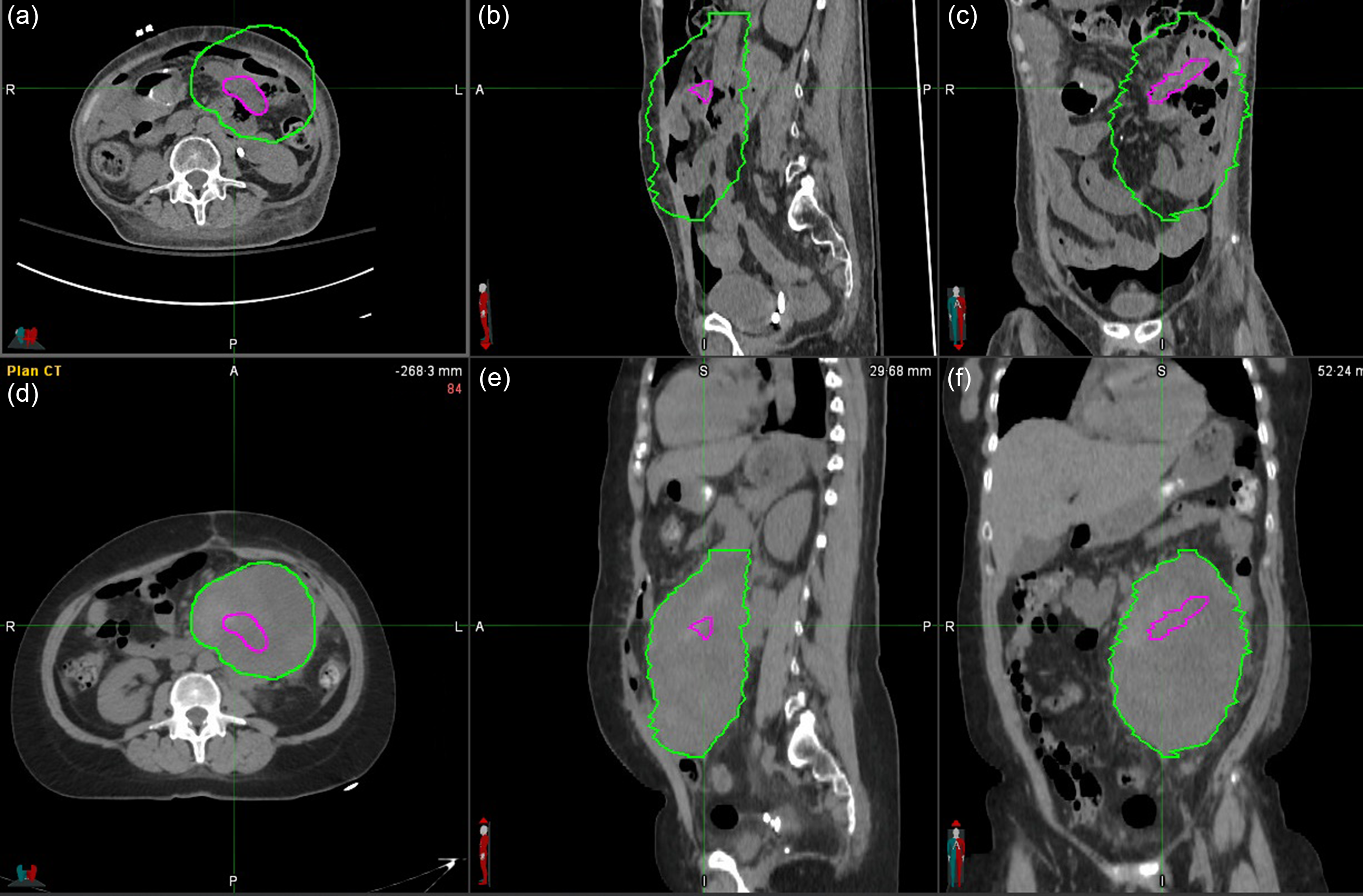
Figure 3. The CTV at treatment (green) and at the 8·5-month follow-up (magenta). Top row: 8·5 month-follow-up CT (A axial, B sagittal and C coronal plane), bottom row: planning CT (D axial, E sagittal and C coronal plane). The volume of the tumour shrunk from 945 to 17 cc.
Discussion
Neither the biological rational behind the favourable outcomes of SFRT nor the optimal dosimetric prescription parameters for the radiation dose distribution are fully understood, Reference Billena, Khan and Current3,Reference Zhang, Wu and Zhang5,Reference Moghaddasi, Reid and Bezak13 nor is there a firm consensus on the best timing of SFRT and EBRT (the four-week interval we employed is representative of current practice). Nonetheless, the use of GRID radiotherapy resulted in effective and sustained palliation of a large uterine leiomyosarcoma in this patient’s case. Although the patient developed new metastasis and died of metastatic progression, the primary tumour site remained controlled and without pelvic symptoms.
GRID is an excellent treatment option for the management of tumours that, due to their size or previous radiation history, cannot be treated with SBRT. It requires only limited resources; inverse treatment planning or sophisticated radiation delivery systems are not required. This is especially beneficial in low- and middle-income countries, where patients can present with large bulky tumours and healthcare resources may be limited, especially in the palliative setting.
While more research is clearly needed, the authors hope it will see wider acceptance in the treatment of large tumours.
Financial support
None.
Competing of Interest
None.







