Introduction
Dose-escalated radiotherapy improves biochemical progression-free survival (bPFS) in localised prostate cancer.Reference Peeters, Heemsbergen and Koper1–Reference Zietman, DeSilvio and Slater4 However, this can be at the expense of increased late rectal and bladder toxicities.Reference Dearnaley, Sydes and Graham2, Reference Kuban, Tucker and Dong3, Reference Syndikus, Morgan and Sydes5 This balance between tumour control and toxicity forms the basis of the therapeutic ratio. One way to improve the therapeutic ratio is with intensity modulated radiotherapy (IMRT), which allows better conformation of radiotherapy to the shape of the target.Reference Alicikus, Yamada and Zhang6–Reference Sharma, Li and Chen7 Thus, the radiotherapy dose can be increased while minimising the dose to the normal organs at risk.
There is evidence that prostate cancer is relatively radioresistant when compared with the surrounding normal tissues. Mirabell et al. calculated an α/β ratio of 1·4 for prostate cancer by analysing the data of over 5,000 patients. If this is true, there should be a biological advantage to treating with a hypofractionated regimen.Reference Miralbell, Roberts, Zubizarreta and Hendry8 Hypofractionation would increase cancer cell death, whereas normal tissue toxicity remains constant.Reference Fowler, Chappell and Ritter9, Reference Brenner, Martinez and Edmundson10 It would also reduce overall treatment time providing benefits in terms of patient acceptability and cost-effectiveness. However, there are limited published outcome and toxicity data for dose-escalated hypofractionated schedules.Reference Kupelian, Thakkar and Khuntia11–Reference Dearnaley, Syndikus and Sumo13 Most studies use clinician-reported outcomes, which can be unreliable when evaluating late effects.Reference West and Davidson14
We have previously reported similar tumour control and normal tissue toxicity in patients treated with 50 Gy in 16 daily fractions (equivalent total dose of 66 Gy, assuming an α/β ratio for prostate cancer of 1·5), with published results from patients treated to a total dose of 65–70 Gy in 1·8–2·0 Gy fractions.Reference Livsey, Cowan and Wylie15 However, the biochemical outcome for patients with intermediate or high-risk disease was inferior to dose-escalated series using 2 Gy per fraction,Reference Pollack, Zagars and Starkschall16 a finding replicated in a subsequent study using hypofractionated radiotherapy to a relatively low total equivalent dose.Reference Higgins, McLaren and Kerr17 Evidence for a dose effect above 70 Gy led to interest in dose-escalated hypofractionated radiotherapy. Results from a phase II clinical trial at our centre demonstrated that using an IMRT technique, dose-escalated hypofractionated treatment was deliverable and well tolerated with minimal toxicity 2 years post treatment.Reference Coote, Wylie and Cowan18 We now present outcome and late toxicity data using a validated patient questionnaireReference Livsey, Routledge and Burns19 for 88 patients with predominantly high-risk prostate cancer treated with 57 Gy in 19 daily fractions (equivalent to a total dose of 73 Gy in 2 Gy fractions assuming an α/β ratio of 1·5), using IMRT.
Materials and methods
The hospital radiotherapy database was searched to find consecutive patients treated for localised prostate cancer with 57 Gy in 19 daily fractions using IMRT between May 2002 and July 2008. All identified patients were included in the analysis. Case notes were retrospectively reviewed to collect baseline patient and disease characteristics as well as outcome and toxicity data. Toxicity was additionally assessed prospectively using a validated patient questionnaire, which was sent to patients and returned by post.
Patient and disease characteristics
Patient age, pre-treatment prostate-specific antigen (PSA), prostate cancer T stage and Gleason score were recorded. Patients were then assigned to low-, intermediate- or high-risk prostate cancer prognostic groups, according to the D'Amico classification system.Reference D'Amico, Schultz and Schneider20
Treatment
All patients received 57 Gy in 19 daily fractions over 25 days (4 weeks, 5 fractions/week). Patients were treated supine with an empty bladder. The radiotherapy computed tomography planning scan was performed in the treatment position from the L5–S1 interface to 10 cm caudal to ischial tuberosities with a slice thickness of 5 mm. Clinical target volume (CTV1) encompassed the prostate and seminal vesicles and CTV2 the prostate alone. The pelvic lymph node regions were not included in the CTV. The outer rectal wall was contoured from the rectosigmoid junction to the anorectal junction and outer bladder wall contoured in its entirety. A planning target volume (PTV) was generated by addition of a 1 cm margin to CTV1 except at the prostate–rectum interface where the margin was 0·7 cm. PTV2 consisted of CTV2 alone without margin. IMRT was inverse-planned, using five isocentric fields with posterior, right lateral oblique, right anterior oblique, left anterior oblique, left lateral oblique fields (180°, 260°, 325°, 35°, 100° fields, respectively). IMRT was delivered using a LINAC with step-and-shoot multi-leaf collimator capability with 8 MV photons, once daily, 5 days/week. Treatment verification was performed using cone-beam scans on days 1–3 and weekly thereafter, unless otherwise clinically indicated. Dose parameters for target volumes and organs at risk have been previously reported.Reference Coote, Wylie and Cowan18
The duration of neoadjuvant and adjuvant hormone therapy was determined by the treating physician and recorded for each patient. In the majority, this was a luteinising hormone-releasing hormone agonist, goserelin acetate 3·6 mg subcutaneously every 28 days with initial anti-androgen cover or, alternatively, anti-androgen therapy alone (bicalutamide 150 mg once daily).
Follow-up, outcomes and toxicity
Patients were reviewed every three months for the first 2 years following treatment. If the PSA remained stable, patients were then seen six monthly for a total of at least 5 years. Outcomes were reported for all patients and for the high-risk subgroup of patients as overall survival, cause-specific survival and bPFS, defined by the Phoenix criteria (failure at nadir PSA +2 ng/ml).Reference Roach, Hanks and Thames21 Toxicity was assessed by Radiation Therapy Oncology Group (RTOG) criteria and collected by retrospective review of case notes.22 Acknowledging that in a retrospective survey, minor late toxicity may not be adequately reported; bowel, urinary and sexual function toxicities were additionally evaluated using a validated Late Effects in Normal Tissues Subjective, Objective, Management and Analytic scales (LENT/SOMA; subjective part) questionnaire, sent to patients and returned by post.Reference Livsey, Routledge and Burns19 Toxicity was reported on a 4-point scale, where a score of 0 represents no toxicity and a score ≥2 denotes toxicity affecting patient quality of life. Median and maximum scores for each symptom category using data returned from patients were presented.
Statistical considerations
Actuarial overall survival, cause-specific survival and bPFS were estimated using the Kaplan–Meier method. Date of death was used or observations censored at the last date that the patient was seen. A univariate Cox regression analysis was performed to identify prognostic factors for cause-specific survival including patient age, pre-treatment PSA, prostate cancer T stage, Gleason score, high-risk disease and treatment with adjuvant hormone therapy.
Results
Eighty-eight men were treated between May 2002 and July 2008. Median and mean follow-up were 32 and 44 months, respectively (range, 3–97 months).
Baseline characteristics
Median patient age was 67 years (range, 50–87 years). Median pre-treatment PSA was 15·0 ng/ml (range, 2·6–54·0 ng/ml). The distributions of prostate cancer T stage, Gleason score, pre-treatment PSA and risk stratification are shown in Table 1. Seventy of eighty-eight patients had high-risk disease.
Table 1 Baseline characteristics
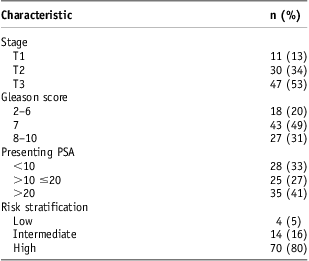
Abbreviation: PSA, prostate-specific antigen.
Hormone therapy
Neoadjuvant and adjuvant hormone therapy were prescribed in 85/88 and 50/88 patients, respectively. Three (3·4%) patients, who all had low risk disease, received no hormone therapy. Of those with high-risk disease, 46/70 were treated with adjuvant hormone therapy. The duration of androgen deprivation therapy is shown in Table 2.
Table 2 Duration of neoadjuvant and adjuvant hormone therapy
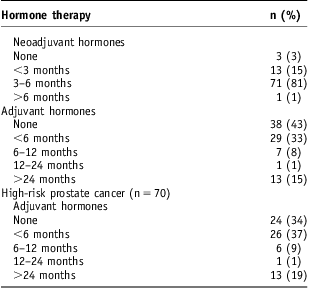
Outcomes
Sixteen patients had died, 11 from prostate cancer and five from an intercurrent cause. Actuarial overall survival rates at 3 and 5 years were 92·5% (95% CI: 82·2–97·0) and 83·5% (68·1–91·9; Figure 1a) and cause-specific survival 95·1% (84·8–98·5) and 88·1% (72·1–95·2), respectively. At three and five years, bPFS were 79·0% (65·0–87·9) and 64·9% (45·1–79·1) (Figure 1b), respectively. For patients with high-risk disease (n = 70), 3 and 5 year actuarial overall survival were 92·8% (81·3–97·1) and 86·0% (70·0–93·8); cause-specific survival 94·2% (82·3–98·2) and 87·3% (71·0–94·8); and bPFS 75·3% (59·7–85·5) and 61·9% (42·4–76·5), respectively.

Figure 1 (a) Kaplan–Meier estimate for overall survival in all patients and (b) Kaplan–Meier estimate for biochemical progression-free Survival (Phoenix definition) in all patients.
None of the factors included in the proportional hazards univariate analysis were significantly associated with cause-specific survival (patient age, p = 0·21; pre-treatment PSA, p = 0·45; prostate cancer T stage, p = 0·65; Gleason score, p = 0·07; high-risk disease, p = 0·43; and use of adjuvant hormone therapy, p = 0·76).
Toxicity
On retrospective review of case-notes, 12 (14%) patients reported RTOG grade I bowel or urinary toxicity, three patients (3%) grade II toxicity and no patients experienced grade III toxicity or above.
LENT/SOMA questionnaires were returned by 53/72 patients. Of those that returned questionnaires, all answered questions about bowel function, 96% regarding urinary symptoms and 92% sexual function. For bowel function, the median toxicity score was <1 (Figure 2). Significant bowel symptoms, defined as a maximum score ≥2 for at least one question, were noted in 64% of patients (Figure 3), and 2/53 reported severe symptoms with a maximum score of 4 (Table 3). The median score for urinary symptoms was also <1. A similar proportion of patients had significant urinary symptoms; 59% recorded a maximum score ≥2 but no patients had a score of 4. For sexual function, the median score was 1·5. Nearly all patients (96%) had significant maximum scores ≥2, and most (57%) recorded a score of 4 for at least one question relating to sexual function.

Figure 2 LENT/SOMA data. Note: Median scores per symptom area (range, 0–4). Abbreviation: LENT/SOMA, Normal Tissues Subjective, Objective, Management and Analytic scales.

Figure 3 LENT/SOMA data. Note: Maximum scores per symptom area, non-significant scores (score 0·1) and significant scores (≥2). Abbreviation: LENT/SOMA, Normal Tissues Subjective, Objective, Management and Analytic scales.
Table 3 LENT/SOMA data
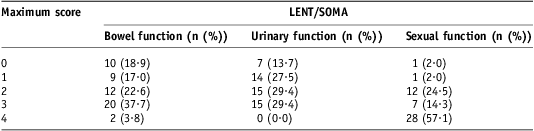
Note: Number of patients with maximum scores per symptom area (percentage of patients in parentheses).
Abbreviation: LENT/SOMA, Late Effects in Normal Tissues Subjective, Objective, Management and Analytic scales.
Discussion
There is considerable interest in dose-escalated hypofractionated radiotherapy in the treatment of localised prostate cancer as treating cancers with a low α/β ratio may increase the therapeutic ratio.Reference Arcangeli, Saracino and Gomellini12 However, there are limited published results comparing hypofractionation with dose-escalated radiotherapy using conventional fractionation. Here we present outcome results for 88 patients (70/88 with high-risk disease) treated with 57 Gy in 19 daily fractions over 4 weeks (5 fractions/week) to an equivalent total dose of 73 Gy using IMRT. We also describe late toxicity data collected prospectively by a validated patient questionnaire, returned by 74% of patients.
For all patients, 3- and 5-year actuarial bPFS were 79% and 65%, respectively (Figure 1b). In patients with high-risk prostate cancer, 3 and 5-year bPFS were 75% and 62%, respectively. Forty-six out of seventy patients with high-risk disease received adjuvant hormone therapy. We recognise that the current standard of care is 2 or 3 years of treatmentReference Bolla, Van Tienhoven and Warde23; but these patients pre-date this era. One difficulty in comparing outcome results between published studies is that there is heterogeneity in treatment with hormone therapy. Nonetheless, our results appear favourable compared with those from randomised studies of hypofractionated radiotherapy, although in these studies patients were treated to a relatively low total equivalent dose.Reference Lukka, Hayter and Julian24, Reference Yeoh, Holloway and Fraser25 Five year outcome data are available from two randomised trials, where patients did not receive adjuvant hormone therapy and results were not stratified by disease prognostic group. In the larger trial, 466 patients with T1-2N0M0 prostate cancer were treated with 52·5 Gy in 20 daily fractions to a total equivalent dose of 62 Gy, using a 2D technique. Overall survival and freedom from biochemical failure (Houston definition) at 5 years were 88% and 58%, respectively.Reference Lukka, Hayter and Julian24 Similarly, Yeoh et al.Reference Yeoh, Holloway and Fraser25 reported 108 patients with T1-2N0M0 prostate cancer treated to a total equivalent dose of 67 Gy mainly using a 2D technique, 5-year overall survival and freedom from failure (biochemical failure defined by Phoenix criteria) of 86% and 57%, respectively. However, follow-up was unsatisfactory and PSA data were only obtained from 98/162 patients who were alive at 5 years. In a large retrospective series of 705 men who received 50 Gy in 16 daily fractions to an equivalent total dose of 66 Gy using a 3D technique and without hormone manipulation, 5-year bPFS were 82%, 56% and 39% in patients with low, intermediate and high-risk disease, respectively.Reference Livsey, Cowan and Wylie15 In a second series, where 300 patients were treated with 52·5 Gy in 20 daily fractions to a total equivalent dose of 62 Gy and with 3 months of neoadjuvant hormone therapy, 5-year bPFS were 74%, 56% and 31% in low, intermediate and high-risk groups.Reference Higgins, McLaren and Kerr17
Our results also appear to be comparable with published outcome data for patients with high-risk disease treated with dose-escalated radiotherapy. A randomised phase III trial of 168 patients with high-risk prostate cancer compared dose-escalated hypofractionated and conventional fractionation radiotherapy using a 3D technique. All patients received 2 months neoadjuvant and 7 months adjuvant androgen deprivation therapy. Eighty-three patients were treated to a total equivalent dose of 80 Gy with 62 Gy in 20 fractions over 5 weeks, 4 fractions/week and 85 patients received conventional fractionation, 80 Gy in 40 daily fractions over 8 weeks. In the hypofractionated arm, 3-year bPFS (Phoenix definition) was 88%.Reference Arcangeli, Saracino and Gomellini12 In a retrospective series of 100 patients treated with hypofractionated radiotherapy to a total equivalent dose of 80 Gy with 70 Gy in 28 daily fractions using IMRT, 5-year bPFS (Phoenix definition) was 88%. In a subgroup of 34/100 patients with high-risk disease, where 91% received androgen deprivation therapy for up to 6 months, the 5-year bPFS was 75%.Reference Kupelian, Thakkar and Khuntia11
Evidence from randomised phase III clinical trials suggests that dose-escalated radiotherapy using conventional fractionation improves local and biochemical disease control.Reference Peeters, Heemsbergen and Koper1–Reference Zietman, DeSilvio and Slater4 Although we take caution in equating results from subgroup analyses of different studies, our results are similar to outcome data for patients with high-risk disease treated in this way. The MD Anderson Cancer Center reported 5-year freedom from failure of 69% (extrapolated from graph, biochemical failure defined by Phoenix criteria) in a high-risk sub-group of 53 patients treated without hormone therapy in the dose-escalated 78 Gy arm.Reference Kuban, Tucker and Dong3 In the Dutch multicentre randomised phase III trial, which allowed hormone therapy, the 5-year freedom from failure (biochemical failure defined by the American Society for Radiation Oncology criteria) was 56% in a subgroup of 177 patients with high-risk disease who received a total dose of 78 Gy.Reference Peeters, Heemsbergen and Koper1 The Medical Research Council (MRC) RT01 trial reported 5-year bPFS of 57% in 184 patients with high-risk disease treated with 3 to 6 months of neoadjuvant hormone therapy and 74 Gy in 37 fractions.Reference Dearnaley, Sydes and Graham2
In this series, late toxicity measured by RTOG criteria was low. Clinician-reported retrospective toxicity data can be unreliable. Seventeen per cent of patients experienced grade I or II bowel or urinary symptoms and no patients experienced grade III toxicity or above. These results are comparable to those seen in studies of patients treated with hypofractionated radiotherapy to a total equivalent dose of 66 Gy using a 3D technique, and 62 Gy using a 2D technique with 3·125 Gy fractions and 2·625 Gy fractions, respectively.Reference Livsey, Cowan and Wylie15, Reference Lukka, Hayter and Julian24 They are also similar with results from dose-escalated hypofractionation studies. In a phase III randomised trial, where patients were treated to an equivalent total dose of 80 Gy with 3·1 Gy fractions over 5 weeks, actuarial 3-year grade II bowel and urinary toxicity were 17% and 16%, respectively and grade III toxicity was seen in only two patients.Reference Arcangeli, Saracino and Gomellini12 Kupelian et al.Reference Kupelian, Thakkar and Khuntia11 treated patients to a total equivalent dose of 80 Gy with 2·5 Gy fractions using IMRT and reported 5-year grade II or III rectal and urinary toxicity of 5% and 8%, respectively. The results also compare favourably with toxicity reported in the dose-escalated arms of phase III clinical trials using conventional fractionation.Reference Peeters, Heemsbergen and Koper1–Reference Zietman, DeSilvio and Slater4 Initial data from the Conventional or Hypofractionated High dose Intensity modulated radiotherapy for Prostate cancer (CHHiP) study supports our data. Dearnaley et al.Reference Dearnaley, Syndikus and Sumo13 have recently published preliminary toxicity data from the first cohort of patients within the CHHiP study comparing the standard fractionation regime with two hypofractionated regimes confirming equivalent toxicities between the three arms. However, we recognise the limitations of our results, which include retrospective collection of data and possible physician underreporting of minor or moderate toxicity. We therefore prospectively assessed toxicity using a validated LENT/SOMA patient questionnaire.
In assessing LENT/SOMA toxicity scores it is important to consider both median and maximum scores as these tend to under and over represent toxicity, respectively. For bowel and urinary symptoms LENT/SOMA median toxicity scores were both <1 (Figure 2), which suggests that overall late effects from radiotherapy were well tolerated. However, 64% and 59% of patients recorded a maximal score of ≥2 for at least one bowel and urinary symptom question, respectively, which reflects that some patients experienced long-term toxicity affecting quality of life. Nearly all patients (96%) had significant maximal toxicity scores ≥2 (Table 3, Figure 3) when recording sexual function. This may partly be due to use of androgen deprivation therapy. One weakness of this LENT/SOMA assessment is the lack of comparison with baseline pre-treatment data. In the United Kingdom, where there is no PSA screening programme, most patients present with symptoms and so these scores may overestimate late toxicity. Indeed, in a phase II study at our centre, which assessed acute and 2-year toxicity of dose-escalated hypofractionated radiotherapy using IMRT in 60 patients, patients reported significant pre-treatment symptoms (LENT/SOMA maximum bowel and urinary symptom scores ≥2 in 25% and 76% of patients, respectively).Reference Coote, Wylie and Cowan18 We also acknowledge the possibility of self-selection bias in return of questionnaires.
It is difficult to compare LENT/SOMA scores from other published studies because of inherent differences in patient populations and the questionnaires are not reported in an identical way. Yeoh et al. assessed toxicity with a modified LENT/SOMA questionnaire in 108 patients treated to a total equivalent dose of 67 Gy in 2·75 Gy fractions, mainly using a 2D technique. At 5 years, 51% and 48% of patients reported bowel and urinary symptoms that adversely affected their daily activities.Reference Yeoh, Botten, Butters, Di Matteo, Holloway and Fowler26 In the dose-escalated arm of the MRC RT01 trial at 5 years follow-up, 47% and 59% of patients recorded maximal LENT/SOMA scores ≥2 for bowel and urinary symptoms, respectively.Reference Dearnaley, Sydes and Graham2 These results are in keeping with our findings and it is evident that some patients experience significant long-term side effects with dose-escalated radiotherapy.
This series confirms that dose-escalated hypofractionated radiotherapy using IMRT is deliverable, with promising outcomes and late toxicity in line with other radiotherapy regimes. Evidence of a low α/β ratio for prostate adenocarcinoma is mounting leading to speculation that hypofractionated radiotherapy may improve the therapeutic ratio while delivering external beam radiotherapy in a way that is advantageous to the patient and health economics. The fractionation schedule reported here is now being compared with conventional dose-escalated treatment within an on-going multicentre phase III clinical trial.Reference Dearnaley, Syndikus and Sumo13
Acknowledgements
The authors thank The Christie Clinical Audit and Outcomes department for supporting data collection and the patients who contributed to the study.
Conflict of interest
None.






