Introduction
Paragangliomas and pheochromocytomas are rare slow-growing benign neuroendocrine tumours. Reference Persky and Tran1 Pheochromocytomas arise within the adrenal glands, whereas paragangliomas are found in the extra-adrenal autonomic paraganglia. Reference Erickson, Kudva and Ebersold2 Both tumours have the ability to secrete catecholamines; however, pheochromocytomas are much more likely to do so. Reference Lenders and Eisenhofer3–Reference Sawka, Jaeschke and Singh5 The majority of paragangliomas arising around the base of skull region are non-secreting tumours. Reference Carlson, Sweeney and Pelosi6 When treating these tumours with radiotherapy, the goal is to achieve local control with the least amount of toxicity to surrounding tissues. Reference Smith, Harvey and Darr7 This goal is even more important in paediatric populations due to the risk of secondary malignancies arising later in life. In this case study, we compared a proton plan to a photon plan for a paediatric patient.
Clinical history
The patient is a 16-year-old boy who presented with worsening headaches, difficulty in swallowing, neck pain, tinnitus and intermittent tongue spasms. The patient also reported pressure behind his eye without any vision changes. Magnetic resonance imaging of the head and neck region demonstrated a cystic mass near the left jugular foramen measuring 1·7 cm x 2·7 cm x 3·5 cm (AP x W x CC). The mass was noted to occupy the left superior parapharyngeal space. Computed tomography of the neck revealed cystic and necrotic characteristics. Contrast enhancement allowed for better visualisation revealing a 3·1 cm x 2·3 cm x 4·6 (AP x W x CC) thick-walled lesion extending from the jugular foramen to C1–C2 level. No metanephrines were noted in a 24-hours urine study.
Multidisciplinary tumour board treatment decision was for radiotherapy because of the location of the lesion and also high surgical risk. Both a proton and a photon arc plan were generated. Due to the patient’s young age, as well as clear superior dosimetric profile, the decision was made to treat the patient with proton beams. The patient was treated with a dose of 5500 cGy (CGE) in 25 fractions, where CGE stands for cobalt Gray equivalent and had overall good treatment tolerance. The patient met with the physician team weekly to discuss ongoing side effects. The patient was noted to have grade 2 skin erythema towards the end of his treatment along with grade 1 dysphagia. The patient also reported that headaches initially became worse during treatment but subsided towards the end. The patient was seen for follow-up at 18 months after completing treatment. The patient denied any residual dysphagia, odynophagia, hoarseness or throat soreness. MRI of the face and neck with and without contrast obtained during that visit was consistent with a significantly smaller mass measuring 1·1 cm x 1·6 cm x 2·7 cm (AP x W x CC).
Discussion
This young boy’s tumour was located near the left jugular foramen. Dose distributions and dose–volume histograms for photon and proton beam are shown in Figures 1 and 2, respectively. As shown in Table 1, the majority of the right-sided structures received a significantly lower dose than left-sided structures in the proton plan when compared to photon plan. The right parotid gland received a mean dose of 1·3 cGy (CGE) and a max dose of 8·7 cGy (CGE) with our proton plan. In the photon plan, the right parotid gland received a mean dose of 386·3 cGy and a max dose of 854·7 cGy. Interestingly, the oral tongue received a higher max dose in the proton plan than photon plan 2704·3 cGy (CGE) versus 2195·4 cGy. The mean dose remained lower in the proton plan versus photon plan at 209·6 cGy (CGE) versus 676·8 cGy, respectively. This finding may likely be attributed to the location of some portions of the oral tongue in relation to the tumour. This level of physical dose sparing allows for dose escalation within the target if necessary or desired while preserving surrounding structures. In this patient’s case, given the benign nature of his condition, it was paramount to limit dose outside of our target as much as possible.

Figure 1. (a) Photon beam dose distribution with glomus tumour noted near the left jugular foramen. (b) Proton beam dose distribution and sparing of contralateral structures.

Figure 2. Dose–volume histogram (DVH) comparing both plans. Structures graphed with triangle are from photon plan. Structures graphed with square are from proton plan. Doses are listed in Table 1.
Table 1. Dose comparison to organs-at-risk for a left-sided glomus tumour in a paediatric patient. Proton doses in cGy (CGE) to contralateral structures are significantly lower than photon doses in cGy
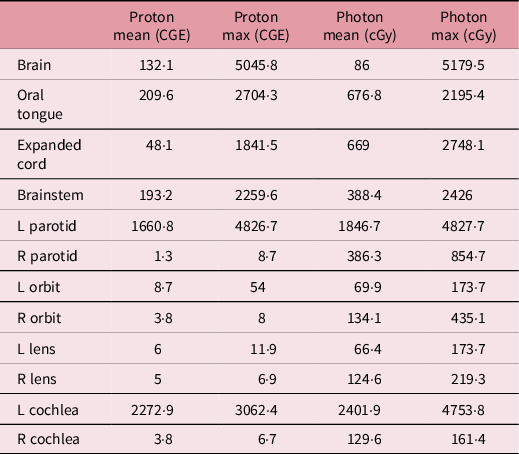
A higher maximum dose was noted in the proton plan of 5586·1 cGy (CGE) versus photon 5349·7 cGy despite identical prescriptions. It is possible that this higher dose was seen either because of the direction of the beams used or a less steep dose fall-off, which could be attributed to the range shifter used for this plan. Paragangliomas are rare tumours that are often described along with pheochromocytomas. These tumours have an estimated annual incidence of 0·8 per 100,000 person years. Reference Beard, Sheps and Kurland8 One of the aspects that makes our case report unique is that most patients are diagnosed with head and neck paragangliomas in their 40 seconds. Reference Al-Harthy, Al-Harthy and Al-Otieschan9 Studies in other malignancies such as rhabdomyosarcoma have also demonstrated that proton therapy provides adequate target coverage while still decreasing mean integral dose. Ladra et al. conducted a phase II clinical trial revealing that the IMRT mean integral dose was 1·8 times higher for H&N (p < 0·01) than proton therapy. Reference Ladra, Edgington and Mahajan10 Current ongoing trials such as DAHANCA 35 (NCT04607694), investigating proton versus photon in head and neck cancers, will provide the medical community with answers regarding proton use in adults. Reference Friborg11 The issue that we often see is that trials like DAHANCA 35 are often focusing on adult patients and exclude paediatric patients. Although important, this trial highlights the lack of higher level evidence for paediatric patients. And given the rarity of paediatric cancers, it is likely that such a trial would have significant challenges in completing accrual. Ioannis et al. published a case series that included 13 adult patients with head and neck paragangliomas that were treated with either proton or photon radiation between 2004 and 2014. Reference Ioannis, Hansen and McDonald12 This retrospective study had a follow-up of 52 months, which is not sufficient when monitoring for long-term complications such as secondary malignancies. This study also did not generate proton plans for its patients who were treated with photons in order to compare dose to surrounding tissues. Chowdhury et al. showed in another case series that both treatment modalities, photons and protons, were equivalent in treating head and neck paragangliomas. Reference Chowdhury, Nead and Lustig13 The median age for this case series was 53. The median follow-up was of 30·9 months.
Conclusion
Given the rarity of paragangliomas in the paediatric population, it is unlikely that a non-inferiority clinical trial of protons versus photons in this patient population will ever come to fruition. This report serves as a dosimetry comparison for a base of skull paraganglioma in a paediatric patient. It provides the medical community with an objective comparison of between two different treatment modalities and provides supporting evidence of physical dose sparing to surrounding organs-at-risk.
Dosimetric superiority of protons in the skull base region is largely due to the absence of dose deposition distal to the target, or ‘exit dose’. This phenomenon is explained by the distinctive Bragg peak that protons have that allows for a rapid dose fall-off beyond the target. Contralateral structures were significantly spared with the proton plan. As previously established, proton beam therapy remains the therapy of choice for paediatric patients given their long-term survival and concerns for secondary malignancy, as well as lower doses to most if not all normal structures of interest.
Acknowledgements
None.






