Introduction
The brachiopod order Atrypida originated during the Ordovician Radiation (sensu Stigall et al., Reference Stigall, Edwards, Freeman and Rasmussen2019) alongside most other brachiopod lineages (Harper et al., Reference Harper, Rasmussen, Liljeroth, Blodgett, Candela, Jin, Percival, Rong, Villas and Zhan2013) and other fossil groups that encompassed the Paleozoic Evolutionary Fauna. Unlike the more well-known atrypides from the Devonian that typically have large, strongly dorsibiconvex shells (such as the namesake of the order, Atrypa), these early atrypides had small ventribiconvex shells and less elaborate spiralia with fewer whorls supporting the lophophore. Because these early species have been understudied, the origin of this important brachiopod order remains uncertain.
One of the most common of these early atrypide genera in Laurentia is Zygospira Hall, Reference Hall1862. The type area of the genus is the upper Katian of the Cincinnati tri-state area (Hall, Reference Hall1862), but species have been reported from rocks of similar age in Ontario (Foerste, Reference Foerste1924), Hudson Bay Lowlands (Jin et al., Reference Jin, Caldwell and Norford1997), Iowa (Wang, Reference Wang1949), and Texas (Howe, Reference Howe1965) (Fig. 1). A number of species reported from older rocks and localities from outside of Laurentia probably belong to other genera, such as the earlier Anazyga Davidson, Reference Davidson1883, or a variety of atrypide genera in the plates that now comprise China and Central Asia that are more distantly related to Zygospira (Copper, Reference Copper1977; Rong et al., Reference Rong, Jin, Shen and Zhan2017). An exception to this is Zygospira carinata Percival, Reference Percival1991, reported from New South Wales (Australia) that extends into the early Katian (although the spiralia in this species has not yet been documented to confirm its assignment to the genus).
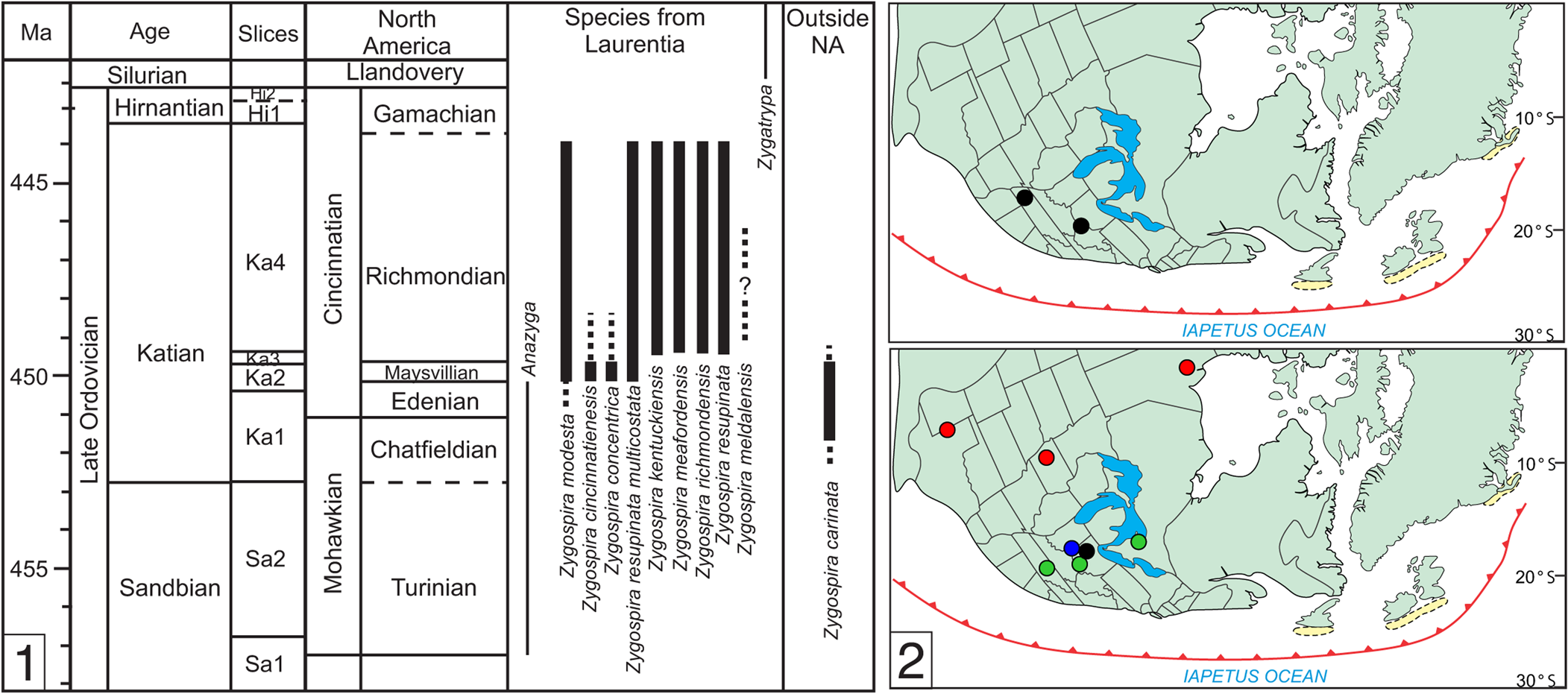
Figure 1. (1) Stratigraphic range of Zygospira and closely related genera Anazyga and Zygatrypa (left). (2) Maps showing the range of Zygospira in eastern North America in the middle (top) to late Katian (bottom). Note that this does not include the numerous species now classified under other atrypide genera or suspected to belong to Anazyga (see Systematic Paleontology). Colors: black = Z. modesta; green = Z. kentuckiensis; blue = Z. cincinnatiensis; red = Z. resupinata and subspecies.
Many species of Zygospira have been defined based on generalized descriptions of differences in shell size and ornamentation but have not been critically re-assessed using contemporary methods. Devising a taxonomic scheme to classify these species is made difficult by the nature of early atrypide morphology and development. For example, unlike other brachiopod orders, such as Orthida or Strophomenida that commonly feature distinctive cardinalia and muscle scars that can be used as diagnostic characters, early atrypides generally have poorly impressed muscle scars and lack distinctive cardinalia and other diagnostic internal structures that have proven useful in distinguishing brachiopod species within other lineages.
The structure of the calcified supports for the lophophore that evolved in the spire bearers (spiralia) has proven to be a useful character for suprageneric classification of the group. Variations in the number of whorls and geometry of the spiralia, as well as variation in structures associated with the spiralia, have been used to differentiate lineages within these early Atrypida (Copper, Reference Copper and Kaesler2002). Unfortunately, many of these features within the shell remain poorly documented due to the labor- and time-intensive process of serial sectioning necessary to produce meaningful cross-sections of these features.
Serial sections illustrate key characteristics of the most common species herein, but the present study focuses on the external morphology of shells to evaluate the existing taxonomic framework of this evolutionary lineage and investigate any functional or paleoecological implications of the morphological disparity present in Zygospira. A new collection of Zygospira kentuckiensis James, Reference James1878, from the upper Katian Queenston Shale near Owen Sound, Ontario, Canada, is described and illustrated, including traced serial sections showing the interior of the shell.
The results of this study will contribute to our understanding of the evolution of the shelly benthos during the Ordovician Radiation. Although the effects of this evolutionary radiation on brachiopod biodiversity are becoming increasingly clear, the underlying processes driving this event are not yet fully understood. Detailed specimen-based analyses such as this may hold the key to understanding the underlying dynamics and evolutionary innovations that drove the rise in biodiversity and morphological disparity during this event.
Previous studies
The genus Zygospira was initially erected by Hall (Reference Hall1862) to include all of the coarsely costate brachiopods with spiralia in the Upper Ordovician rocks of North America based on the earlier described Atrypa modesta Say in Hall, Reference Hall1847. In the initial description of the genus, he rightly recognized the significance of the spiralium as a diagnostic character, noting the presence of a strong loop that differentiates these species from later Atrypida. The similarities in ventral position of the bases of the spiralia were used to hypothesize that these early forms were distant ancestors to the later atrypides of the Silurian and Devonian. Hall (Reference Hall1862) also noted the distinctive external form of these earlier atrypides in comparison to later species, but never elaborated on this point.
Hall and Clarke (Reference Hall and Clarke1894) summarized much of the work done on the genus in the latter part of the nineteenth century. Notably, they synonymized the species assigned to Anazyga Davidson, Reference Davidson1883, with Zygospira based on the similar apparent variability in the spiralia and range in shell shapes of species assigned to both genera. Rather than two separate genera, they saw Anazyga and Zygospira as two endmembers on a continuous spectrum.
Since these earlier studies, few others have attempted a comprehensive study of the early atrypides in Laurentia. Copper (Reference Copper1977) proposed that Zygospira should be divided into two main groups, restoring Zygospira and Anazyga as separate genera. Zygospira sensu stricto included species similar to the type species Zygospira modesta that are strongly ventribiconvex and generally larger in size. These species have spiralia with spires that are dorso-medially oriented within the mantle cavity and have a jugum that connects the spires together high in the dorsal valve posterior to the apices of the spiralia.
Species restored to Anazyga were slightly older, common in the lower Katian (an interval referred to as the Trentonian in North America) of eastern North America, while Zygospira is largely confined to the middle to upper Katian. Anazyga species are united in having spiralia with medial-oriented spires in comparison to the dorso-medial spiralia of Zygospira. Although both genera possess a jugum, Copper (Reference Copper1977) recognized a consistent difference in its location within the shell. Anazyga, so far as is known, typically have a jugum closer to the anterior than in Zygospira, although this needs to be studied in detail to determine how stable this position is among species of the genus. Variability in the shape and configuration of this structure within this lineage remains poorly studied, however. Externally, Anazyga tends to be more biconvex than the later Zygospira species and has a more strongly carinate ventral valve with more prominently differentiated mid and lateral ribs.
Species of Zygatrypa are apparently separated from the genera of the Anazygidae by a stratigraphic gap in the Silurian (Copper, Reference Copper1977). This may be an example of the Lazarus Effect, or this genus may be more closely related to one of the other atrypide lineages from this time. These species are only poorly documented and do not occur as widely as earlier forms.
Although Zygospira was once thought to be a cosmopolitan genus, reviews of atrypide brachiopods from Central Asia (Popov et al., Reference Popov, Nikitin and Sokiran1999) and China (Rong et al., Reference Rong, Jin, Shen and Zhan2017) have revealed that species from these plates and terranes mostly belong to the Atrypinidae rather than the Anazygidae. Late Ordovician Atrypinidae usually have a dorsal fold and ventral sulcus rather than the dorsal sulcus and ventral fold of Anazygidae and are so far only known to possess separated jugal processes rather than one solid jugum connecting the spiralia. The functional significance of these differences remains uncertain, but Zygospira sensu stricto are now almost entirely known from Laurentia. The only known occurrence of the genus as it is currently defined outside of Laurentia is Zygospira carinata Percival, Reference Percival1991, from the Upper Ordovician of New South Wales, Australia, but no information on its spiralia is available to confidently assign the species to Zygospira.
Materials and methods
Several species of Zygospira were examined from collections made by Sproat and Dr. Jisuo Jin (Western University) now deposited at the Royal Ontario Museum (ROM). These were supplemented with specimens from the Geological Survey of Canada (GSC), Cincinnati Museum Center (CMC), Field Museum (UC, IP), American Museum of Natural History (AMNH), and the University of Iowa (SUI).
Diagnostic measurements of well-preserved specimens were recorded using a set of digital calipers and a protractor. These measurements included length, width, and depth of the shell in addition to length of the dorsal valve, depth of each valve measured from the posterior part of the commissure, depth of the deviation of the commissure at the anterior caused by the dorsal sulcus and ventral fold, apex angle formed by the ventral umbo, and number of ribs on the ventral valve (Fig. 2). Care was taken to avoid measuring shells with obvious signs of damage or deformation (e.g., cracks, parts of the shell missing, high degree of asymmetry that could indicate post-depositional deformation, etc.).
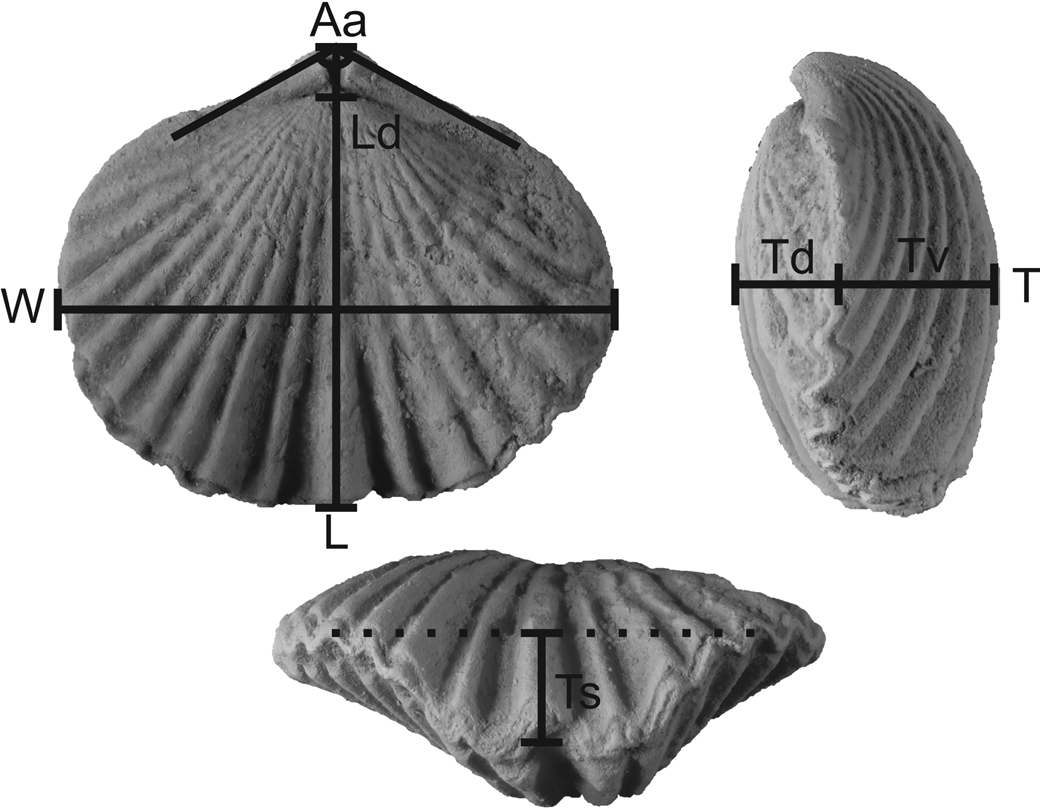
Figure 2. Measurements of Zygospira species in this study. L = length (equivalent to length of ventral valve); Ld = length of dorsal valve; W = width; T = thickness (depth) of specimens; Td = thickness of dorsal valve; Tv = thickness of ventral valve; Ts = depth of the sulcus at the anterior commissure; Aa = apical angle (angle formed by the ventral umbo as it projects across the hinge line). Number of ribs on the ventral valve also was counted.
Measurement data were analyzed using PAST v.4.09 (Hammer et al., Reference Hammer, Harper and Ryan2001). Linear regressions were plotted using the reduced major axis method. Principal component analysis used a correlation matrix that standardizes data to account for differences in the dimensions measured. Discriminant analysis was carried out as a further test of the interspecific variation in morphology.
To examine the internal morphology of shells, brachiopods were serial sectioned using a Croft Parallel grinder. This process involves grinding the fossil down in set intervals and replicating each polished surface with cellulose acetate replication film after a brief rinse with dilute (2–3%) hydrochloric acid (HCl) to expose the shell from the micritic matrix material that fills the mantle cavity. Sections were made every 0.05–0.1 mm and digitally traced using the CorelDraw Graphics Suite.
Repositories and institutional abbreviations
Specimens studied are deposited at the American Museum of Natural History (AMNH), Cincinnati Museum Center (CMC), Field Museum (FM or UC), Geological Survey of Canada in Ottawa (GSC), and Royal Ontario Museum (ROM).
Systematic paleontology
Class Rhynchonellata Williams et al., Reference Williams, Carlson, Brunton, Holmer and Popov1996
Order Atrypida Rzhonsnitskaya, Reference Rzhonsnitskaya and Sarytcheva1960
Suborder Anazygidina Copper in Copper and Gourvennec, Reference Copper, Gourvennec, Copper and Jin1996
Superfamily Anazygoidea Davidson, Reference Davidson1883
Family Anazygidae Davidson, Reference Davidson1883
Subfamily Anazyginae Davidson, Reference Davidson1883
Zygospira Hall, Reference Hall1862
Type species
Producta modesta Say in Hall, Reference Hall1847.
Other species
Copper (Reference Copper1977) briefly reviewed species assigned to Zygospira and restored Anazyga Davidson, Reference Davidson1883, to include species with smaller, medially directed spiralia. Using the shell morphology and stratigraphic ranges of the type species as a guide, he then assigned described species of Zygospira either to Zygospira or Anazyga. Some ambiguity remains regarding the spiralia in many species due to the time-intensive nature of serial sectioning necessary to examine the internal morphology of the shells and the lack of density contrast between the shell material and surrounding matrix that makes imaging using modern CT techniques difficult to impossible. That said, several species can confidently be assigned to Zygospira based on their ventribiconvex lateral profile, wider than long outline, strong simple ribs, and known configuration of the spiralia. These include:
Zygospira modesta (Say in Hall, Reference Hall1847).—Say in Hall (Reference Hall1847, p. 141–142, pl. 33, fig. 15); type specimens initially identified as Producta modesta by Say (see Hall, Reference Hall1847).
Zygospira kentuckiensis Nettelroth, Reference Nettelroth1889.—Nettelroth (Reference Nettelroth1889, p. 138–139, pl. 34, 21–23; not pl. 24, 25).
Zygospira resupinata Wang, Reference Wang1949.—Wang (Reference Wang1949, p. 18–19, pl. 10A, figs. 1–12); only a holotype and a single paratype remain in the SUI collections but Jin et al. (Reference Jin, Caldwell and Norford1997) illustrated a specimen of the subspecies Zygospira resupinata multicostata Howe, Reference Howe1965, that clearly possesses dorso-medially directed spiralia consistent with Zygospira (see Jin et al., Reference Jin, Caldwell and Norford1997, pl. 30, fig. 21).
Several species can be synonymized with the above species based on strong similarities in shell morphology including:
Zygospira concentrica Ulrich, Reference Ulrich1879.—Ulrich (Reference Ulrich1879, p. 14, pl. 7, figs. 10a, b); likely a synonym of Z. modesta.
Zygospira meafordensis Foerste, Reference Foerste1924.—Foerste (Reference Foerste1924, p. 125, pl. 15, figs. 3a–c); herein considered a subspecies of Z. kentuckiensis.
Zygospira raymondi Foerste, Reference Foerste1924.—Foerste (Reference Foerste1924, p. 127, 128); not figured, but based on specimens described as Zygospira uphami by Raymond (Reference Raymond1921); likely a synonym of Z. kentuckiensis with a slightly flattened ventral valve in comparison to the type collection.
Zygospira richmondensis Caley, Reference Caley, Wilson, Caley, Sproule and Okulitch1936.—Caley (Reference Caley, Wilson, Caley, Sproule and Okulitch1936, p. 60, 78, pl. 1, figs. 4, 6); likely a synonym of Z. kentuckiensis, although more evenly convex than typical Z. kentuckiensis.
Most of the early (early Katian, pre-Maysvillian) species previously assigned to Zygospira have been reassigned to Anazyga (see also Copper, Reference Copper1977, for a list with minor differences of opinion). Their taxonomic assignment should be critically reassessed after their spiralia and associated structures have been documented. These are not examined in detail here, but include:
Atrypa recurvirostra Hall, Reference Hall1847.—Type species of Anazyga, sometimes referred to in literature as Zygospira recurvirostris.
Zygospira calhounensis Fenton and Fenton, Reference Fenton and Fenton1922.—Fenton and Fenton (Reference Fenton and Fenton1922, p. 76–77, pl. 2, figs. 4–6).
Zygospira circularis Cooper, Reference Cooper1956.—Cooper (Reference Cooper1956, p. 670, pl. 141C, figs. 18–21, pl. 142B, figs. 6–10, pl. 142D, fig. 16).
Zygospira elongata Cooper, Reference Cooper1956.—Cooper (Reference Cooper1956, p. 670–671, pl. 268G, figs. 29–32).
Zygospira gutta Oraspõld, Reference Oraspõld1956.—Oraspõld (Reference Oraspõld1956, p. 64–65, pl. 4, figs. 14, 15).
Zygospira lebanonensis Cooper, Reference Cooper1956.—Cooper (Reference Cooper1956, p. 671–672, pl. 142C, figs. 11–15).
Zygospira matutina Cooper, Reference Cooper1956.—Cooper (Reference Cooper1956, p. 672, pl. 141B, figs. 13–17).
Zygospira maynei Roy, Reference Roy1941.—Roy (p. 102–103, fig. 69).
Zygospira mediocostellata Cooper, Reference Cooper1956.—Cooper (Reference Cooper1956, p. 672–673, pl. 143D, figs. 13–18).
Zygospira recurvirostris aequivalvis Twenhofel, Reference Twenhofel1928.—Twenhofel (Reference Twenhofel1928, p. 214, pl. 19, figs. 10–12).
Zygospira recurvirostris noquettensis Hussey, Reference Hussey1926.—Hussey (Reference Hussey1926, p. 162–163, pl. 11, figs. 1–3).
Zygospira recurvirostris turgida Foerste, Reference Foerste1917.—Foerste (Reference Foerste1917, p. 103, pl. 5, fig. 15 a–c).
Zygospira variabalis Fenton and Fenton, Reference Fenton and Fenton1922.—Fenton and Fenton (Reference Fenton and Fenton1922, p. 75–76, pl. 2, figs. 7–9).
Zygospira variabalis fountainensis Fenton and Fenton, Reference Fenton and Fenton1922.—Fenton and Fenton (Reference Fenton and Fenton1922, p. 76, pl. 2, figs. 1–3).
A few species previously assigned to Zygospira share similarities with other atrypide genera or have already been assigned to other genera, including the species below.
Athyris headi Billings, Reference Billings1862.—See Meek (Reference Meek1873, p. 127, pl. 11 a–d) for description of species as Zygospira; assigned to Catazyga as type species by Hall and Clarke (Reference Hall and Clarke1894).
Zygospira uphami Winchell and Schuchert, Reference Winchell, Schuchert, Lesquereux, Woodward, Thomas, Schuchert, Ulrich and Winchell1893.—Convex profile suggests affinities with Catazyga, but spiralia shape and configuration remain unknown.
Zygospira maynei Roy, Reference Roy1941.—See Bolton (Reference Bolton, McCracken and Bolton2000); very convex profile suggests affinities with a different lineage. Idiospira was suggested by Bolton, but the illustrated specimen would be unusual for Idiospira. It seems likely that the shells described by Roy (Reference Roy1941) belong to a slightly older collection and may be Anazyga while the shells described and figured by Bolton (Reference Bolton, McCracken and Bolton2000) are an unusual species of Zygospira or a different lineage altogether.
Zygospira putilla Hall and Clarke, Reference Hall and Clarke1894.—Hall and Clarke (Reference Hall and Clarke1894, p. 157, fig. 150, pl. 54, figs. 35–37; not plate 55 as indicated in text); considered Eospirigerina by Amsden (Reference Amsden1974, p. 72), the elongate shell form would be unusual for Zygospira and possesses a distinctive plate in the interior (see Amsden, Reference Amsden1974, text-figs. 49, 50) not known in other Zygospira species and more variation in rib bifurcation (Amsden, Reference Amsden1974, text-fig. 48).
The affinities of the following species remain uncertain because they are only known from a few specimens, sometimes are poorly preserved, and/or their external morphology is unusual for the genus.
Zygospira sulcata Howe, Reference Howe1965.—Howe (Reference Howe1965, p. 655–656, pl. 81, figs. 9–12); fine ribs indicate affinities with Anazyga, but species is known only from poorly preserved fragmentary material.
Zygospira tantilla Bradley, Reference Bradley1921.—Also resembles Anazyga in shape, but if specimens are truly Richmondian in age, this is by far the youngest known species of Anazyga.
Two species of Zygospira from Scotland and Norway cannot be confidently assigned to Zygospira and require further material to make any definitive assignment.
Zygospira orbis Reed, Reference Reed1917.—Reed (Reference Reed1917, p. 944, pl. 24, figs. 24–27); was assigned to Zygospira from Scotland (part of Laurentia during the Ordovician) alongside an unnamed questionably assigned specimen (Zygospira?, sp. Reed, Reference Reed1917, pl. 24, figs, 28, 29). Described to have a broad dorsal depression on the ventral valve and a fold containing two ribs on the dorsal valve, suggesting affinities with Zygospira. The questionably assigned specimens are unusually elongate for Zygospira, however, and may belong to the Atrypinidae. Additional material will need to be examined before anything definitive can be said about either species.
Zygospira meldalensis Reed, Reference Reed and Kiarer1932.—Reed (Reference Reed and Kiarer1932, p. 144, pl. 22, figs. 12, 12a); from the Upper Hovin Group of Norway, only known from a single poorly preserved specimen that cannot definitively be located, although a single specimen within a limestone fragment bearing the same specimen number contains an external mold of the specimen consisting of only the anterior was described by Neuman et al. (Reference Neuman, Bruton and Pojeta1997); however, the configuration of the brachidial structures remains unknown; resembles Z. kentuckiensis in size and shape.
Several species from the Kazakh terranes and North and South China previously have been assigned to Zygospira, but these have almost all since been reassigned to other genera. These all differ from Zygospira in lacking a complete jugum, although the spiralia of many species are still inadequately known. Most also possess a ventral sulcus and dorsal fold rather than the ventral fold and dorsal sulcus that is typical of Zygospira. These species include:
Zygospira parva Rukavishnikova, Reference Rukavishnikova1956.—Rukavishnikova (Reference Rukavishnikova1956, p. 162–163, pl. 5, figs. 14–16); assigned to Schachriomonia by Popov et al., Reference Popov, Nikitin and Sokiran1999.
Zygospira qinghaiensis Xu in Jin et al., Reference Jin, Ye, Xu and Sun1979.—Xu in Jin et al. (Reference Jin, Ye, Xu and Sun1979, p. 108, pl. 21, figs. 10–15, 19–21, text fig. 51; see also Rong et al., Reference Rong, Jin, Shen and Zhan2017, p. 188).
Zygospira shaanxiensis Fu, Reference Fu1982.—Fu (Reference Fu1982, p. 145, pl. 39, fig. 9a–c; see also Rong et al., Reference Rong, Jin, Shen and Zhan2017, p. 192).
Zygospira (Kuzgunia) bankanasensis Klenina, Nikitin, and Popov, Reference Klenina, Nikitin and Popov1984.—Klenina et al. (Reference Klenina, Nikitin and Popov1984, p. 116); assigned to Sulcatospira by Popov et al., Reference Popov, Nikitin and Sokiran1999.
Zygospira (Sulcatospira) plicata Xu in Jin et al., Reference Jin, Ye, Xu and Sun1979.—Xu in Jin et al. (Reference Jin, Ye, Xu and Sun1979; see also Rong et al., Reference Rong, Jin, Shen and Zhan2017, p. 190).
Zygospira carinata Percival, Reference Percival1991 (p. 165, 167, 169; figs. 20, 27) is one of the few probable Zygospira species from outside Laurentia. It is unusual for the genus in that it has a prominent ventral medial rib rather than an interspace, and generally has fewer and coarser ribs. The shell of this species is more elongate than typical Zygospira, which are usually shorter in length than width. The spiralia and associated structures remain unknown for this species, so it can only be assigned to the genus provisionally until additional specimens with intact shell interiors are found. The unusual morphology may reflect the relative isolation of Australia (part of Gondwana in the Ordovician) from Laurentia and lends the species considerable paleobiogeographic significance.
Zygospira modesta kagawongensis Caley, Reference Caley, Wilson, Caley, Sproule and Okulitch1936 (Caley, Reference Caley, Wilson, Caley, Sproule and Okulitch1936, p. 58) was published without description or figures and thus should be considered a nomen nudum (see also Copper, Reference Copper1977). A search of the database of the Royal Ontario Museum where the other specimens described by Caley (Reference Caley, Wilson, Caley, Sproule and Okulitch1936) were deposited revealed no results.
Diagnosis
See Copper, Reference Copper and Kaesler2002.
Occurrence
Common in middle to upper Katian (Maysvillian–Richmondian) rocks of eastern North America, but its range outside of Laurentia is limited. Species from lower Katian strata are almost certainly Anazyga or another early atrypide genus, but need re-examination.
Remarks
Zygospira has referred to a number of different species in literature. Here, the Treatise on Invertebrate Paleontology (Copper, Reference Copper and Kaesler2002) is followed in referring to only the coarsely ribbed species with mediodorsally directed spiralia. Other authors have included species more recently considered to belong to Anazyga (see above). These species generally have spiralia directed medially rather than dorso-medially (although the internal shell morphology has not yet been documented for all species) and can be recognized externally by a generally more convex dorsal valve, weaker ribs, and often is significantly smaller. Zygospira also characteristically has one or two very prominent ribs on each side of the ventral fold, a feature that is usually absent from species that tentatively have been assigned to Anazyga.
Anazyga has been reported only from rocks that are older than Zygospira, being particularly abundant in the lower Katian Trenton Limestone and equivalent strata in eastern North America (Chatfieldian and Maysvillian in North American terminology). The species referred to Zygospira above are all middle to late Katian (Maysvillian–Richmondian in North American terminology) in age. This led Copper (Reference Copper1977) to suggest that Anazyga was a likely ancestor to Zygospira, but this hypothesis has yet to be tested in a broader cladistic analysis of the lineage.
The paleoecology of Zygospira and other early atrypides has not yet been examined in detail (although see Copper, Reference Copper1977, for a brief discussion), but the genus is known to occur in dense clusters (Fig. 3). These dense accumulations could reflect an opportunistic life habit where Zygospira may have been able to multiply rapidly and take advantage of regular disruptions in the environment, such as regular storm events in eastern North America during the Ordovician (e.g., Brookfield and Brett, Reference Brookfield and Brett1988; Kerr and Eyles, Reference Kerr and Eyles1991; Jennette and Pryor, Reference Jennette and Pryor1993). Alternatively, these dense shell beds could represent periods of quiescence with reduced sedimentation that enabled Zygospira to proliferate (Dattilo et al., Reference Dattilo, Brett, Tsujita and Fairhurst2008, Reference Dattilo, Brett and Schramm2012). McFarland et al. (Reference McFarland, Westrop and Cheel1999) analyzed shell beds in the lower Katian Verulam Formation of Ontario where the older atrypide Anazyga is known to be locally abundant and concluded that most of the shell beds must have been formed from allogenic processes such as winnowing by storms. They mentioned that the dense accumulations of atrypide shells may have been at least partially derived from autogenic processes given their common preservation in apparent life position.
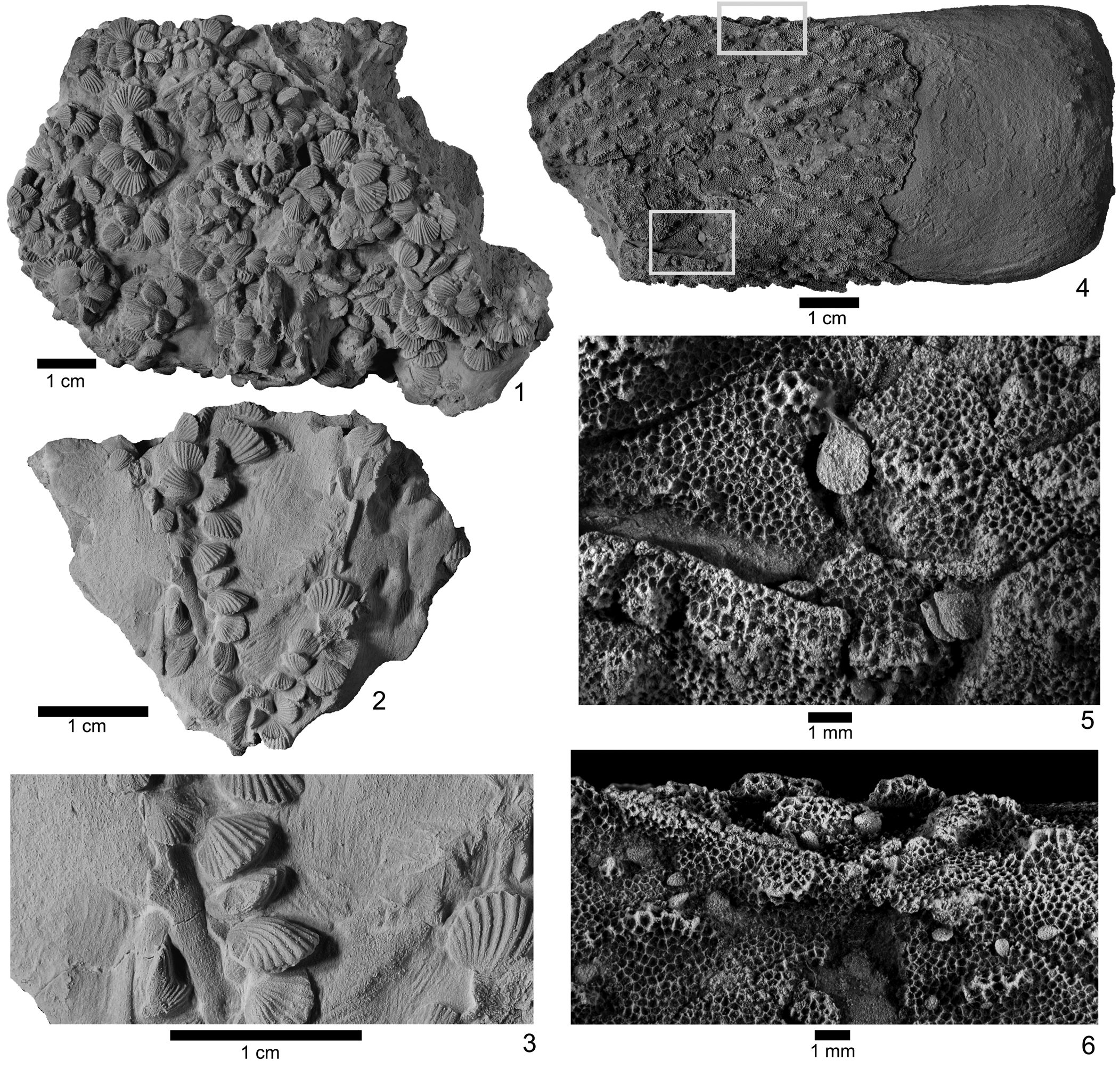
Figure 3. Zygospira modesta attached to bryozoans from Tanner's Creek Formation in Indiana. (1) PE 16630, dense cluster of Zygospira modesta from near Brookville; (2, 3) FM PE16583 Zygospira modesta preserved attached to bryozoan; (4–6) FM PE16631 Zygospira modesta attached to Spatiopora corticans (Nicholson, Reference Nicholson1874) from near Clifton; (5) and (6) magnified views of (4) in blue and green, respectively. Scale bars for (1–4) = 1 cm; (5, 6) = 1 mm.
These dense clusters are also sometimes associated with filter feeders, such as bryozoans and crinoids (Fig. 3). The smaller Z. modesta are preserved in positions that suggest that they may have been attached to these filter feeders when alive via their pedicle (Fig. 3.2, 3.3). There is no evidence of such associations with the larger Zygospira species thus far, however, and the extent of this phenomenon across the Anazygidae has not yet been investigated.
Zygospira modesta Say in Hall, Reference Hall1847
Figures 4, 5
- Reference Hall1847
Atrypa modesta Say in Hall, p. 141, pl. 33, fig. 15a, b; not pl. 33, fig. 15c.
- Reference Hall1862
Zygospira modesta; Hall, p. 155, text-figs. 1, 2.
- Reference Ulrich1879
Zygospira concentrica; Ulrich, p. 14, pl. 7, figs. 10, 10a, b.
- Reference Hall and Clarke1894
Zygospira modesta; Hall and Clarke, pl. 54, figs. 7–10, 12.
- Reference Foerste1910
Zygospira modesta; Foerste, p. 29, pl. 2, fig. 15a, b.
- Reference Foerste1924
Zygospira modesta; Foerste, p. 127, pl. 10, fig. 21a, b.
- Reference Copper1977
Zygospira modesta; Copper, p. 303, pl. 37, figs. 1–8, text-figs. 3, 4.
- Reference Howe1988
Zygospira modesta; Howe, p. 205, fig. 2.1–2.7.
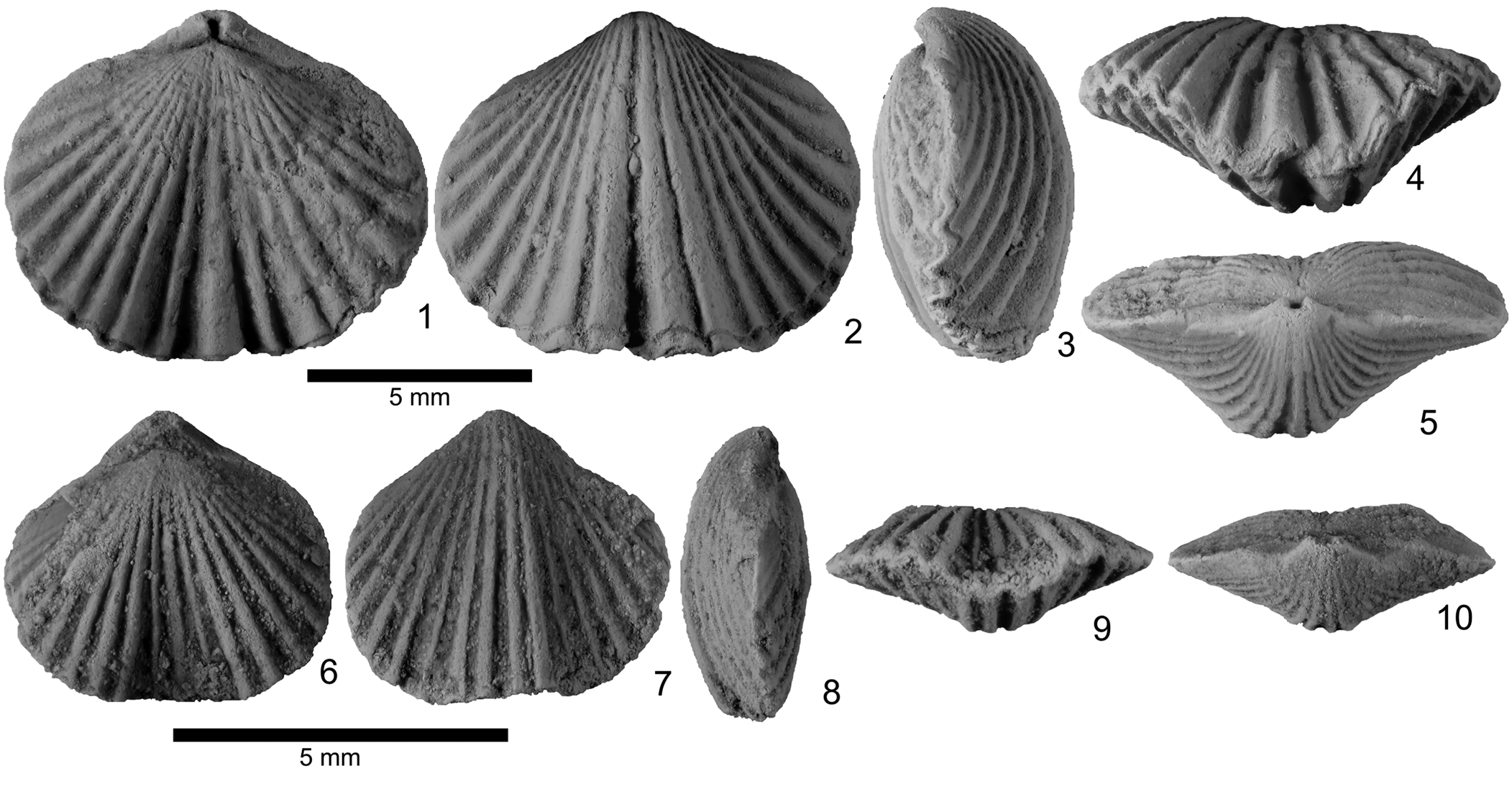
Figure 4. (1–10) Zygospira modesta types from the Hudson River Group (Katian) near Cincinnati, Ohio. (1–5) Lectotype AMNH 29835, dorsal, ventral, lateral, anterior, and posterior views; (6–10) paratype AMNH 29836, dorsal, ventral, lateral, anterior, and posterior views. Scale bars = 5 mm. This is part of Hall's type collection at the AMNH along with several unfigured paratypes in the collection (labeled AMNH 1356d–o).
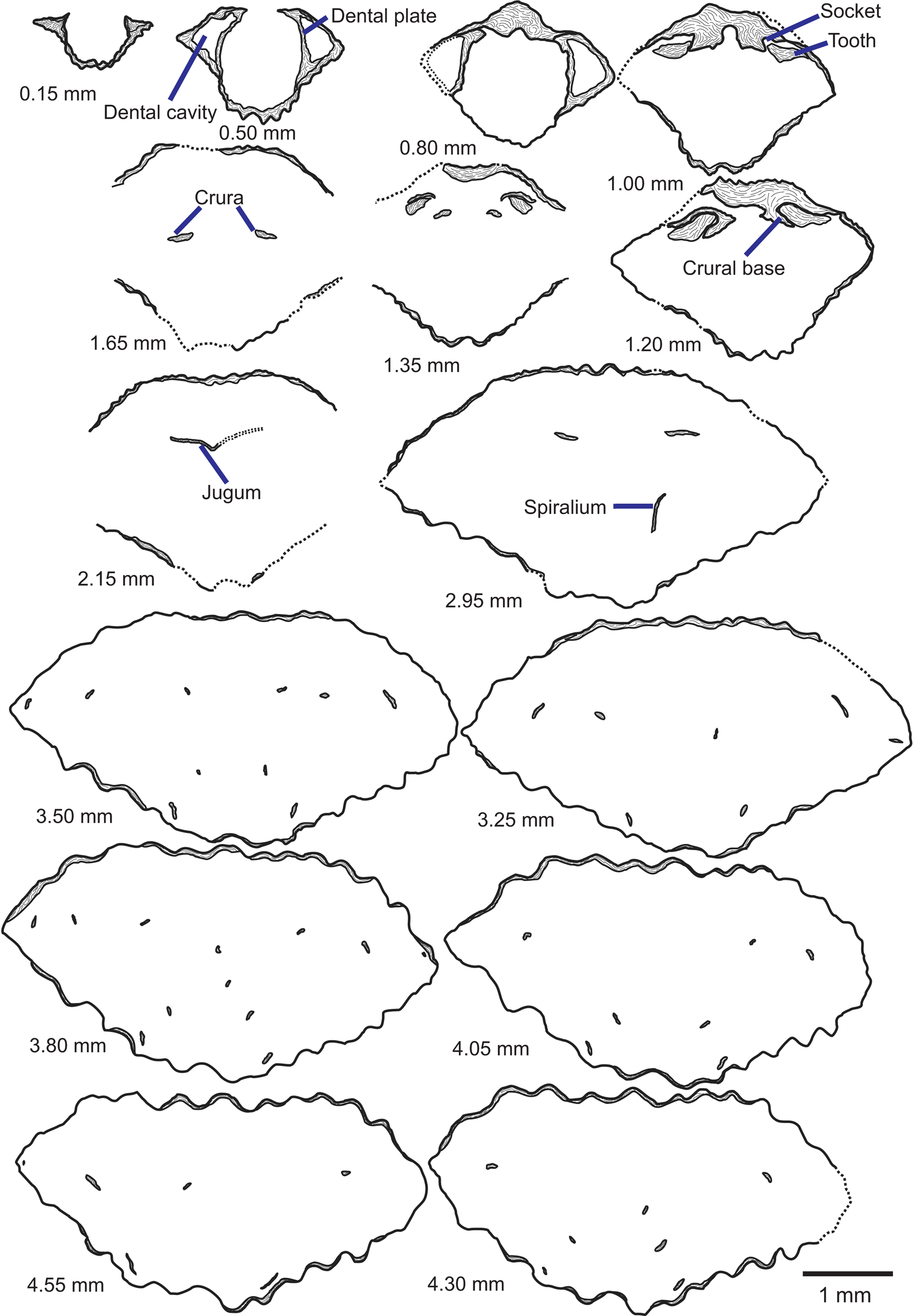
Figure 5. Tracings of serial sections of a Zygospira modesta shell from the Grant Lake Formation near Sharonville in the region around Cincinnati, Ohio (CMC IP96903). Numbers represent distance from posterior of ventral umbo in mm at which the section was ground. Scale bar = 1 mm.
Types
Lectotype AMNH 29835 (formerly AMNH 1356A) selected by Foerste (Reference Foerste1910) from Hall's collection at the AMNH (Fig. 3, AMNH 1356a–d). The specimen tags associated with the type collection lack specific information on the location of the original type locality and strata from which the specimens were collected. The older, typed tags indicate that specimens were collected from the Hudson River Group at Cincinnati, Ohio. These are unlikely to be the original tags, however, because they refer to the specimens as Zygospira while Hall (Reference Hall1847) described the specimens as a species of Atrypa. The newer tags suggest that the specimens are from the Trenton Limestone, but this is highly unlikely. Foerste (Reference Foerste1910) suggested that similar specimens are known from the Fairmount beds near Hamilton, Ohio, which should be considered the type locality. Although this is a reasonable inference, in the absence of any further information regarding their origin, all that can be said is that the types were likely, but not definitively, collected from Cincinnatian-aged strata in the area around Cincinnati.
One of the types, now labeled AMNH 29837 but formerly labeled AMNH 1356C, is characteristic of Z. cincinnatiensis (see below). We tentatively assign it to that species, but a more extensive review is needed to determine the full range of morphological variability within Z. cincinnatiensis, along with a more extensive examination of the internal morphology of both species.
There are 12 unfigured paratypes deposited in the collection along with 2 casts under the collection number AMNH 1356d–1356o (probably following the original numbering scheme—no updated numbers are associated with the specimens).
Diagnosis
See Copper, Reference Copper and Kaesler2002.
Occurrence
Zygospira modesta is most common in middle Katian (Maysvillian) strata of eastern North America near Cincinnati in Ohio, Kentucky, Indiana, and Illinois, but has been reported from upper Katian (Richmondian) strata as well. It is considered the most widespread species of Zygospira, but other species are commonly misidentified as Z. modesta in historical collections.
Description
Shell small, measuring on average 6.4 mm in length, 7.4 mm in width, and 3.7 mm in depth (Fig. 6, Table 1); outline subpentagonal and always significantly wider than long (mean of 87% as long as wide) with ventribiconvex lateral profile with ventral valve ~1.5 times as deep as dorsal valve. Astrophic hingeline with rounded lateral flanks. Anterior margin strongly unisulcate forming prominent flat-topped tongue. Ribs strong and rounded, simple with rare bifurcations and expanding towards anterior, numbering between 14–20, but usually 16–18. Fine growth lamellae on well-preserved specimens cover the shell.
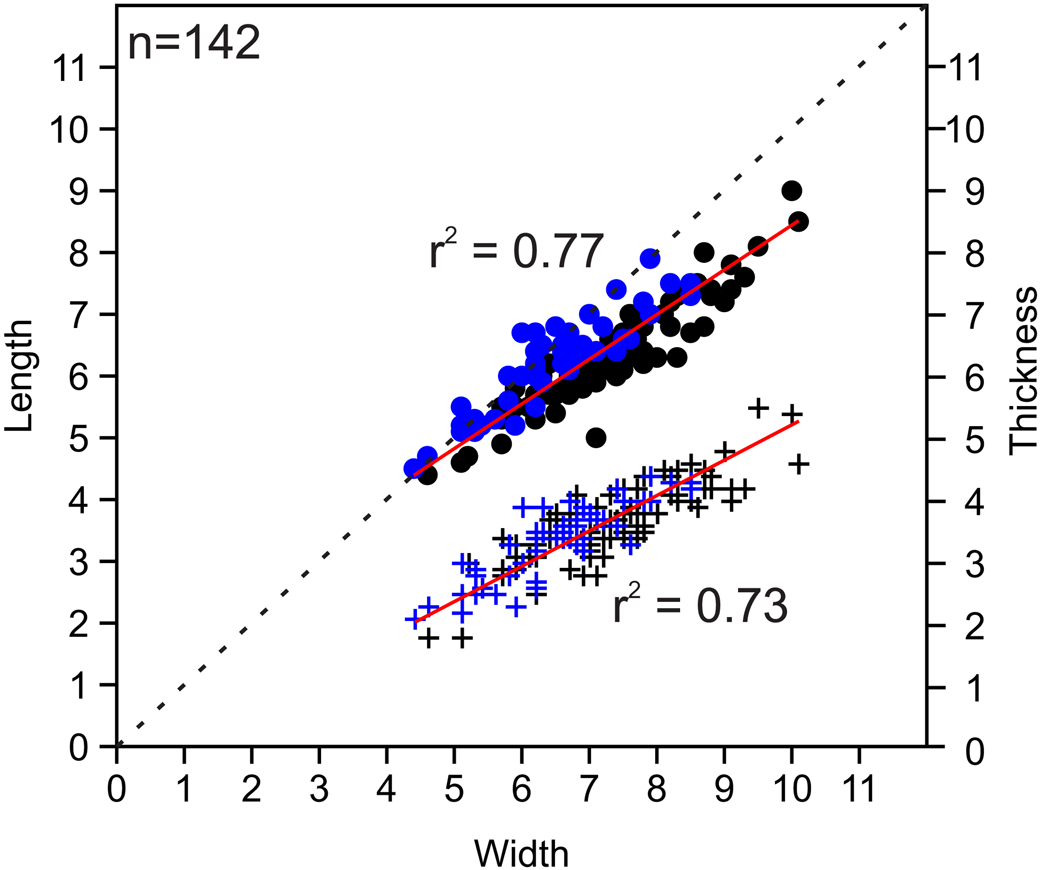
Figure 6. Bivariate plots of length vs. width and thickness vs. width for measured Zygospira modesta specimens. Black = middle Katian (Maysvillian) specimens (n = 92); blue = late Katian (Richmondian) specimens (n = 50); circles indicate length vs. width; plus signs represent thickness vs. width. The r2 values shown (red lines) represent values for combined ages; r2 values for individual datasets are as follows: length/width Maysvillian = 0.85759; thickness/width Maysvillian = 0.70637; length/width Richmondian = 0.82215; thickness/ width Richmondian = 0.75109.
Table 1. Summary statistics for Zygospira modesta specimens measured for this study (n = 92). L = length; W = width; T = thickness (depth); Ad = deflection at anterior of the commissure (all in mm); Aa = apical angle in degrees; R = number of ribs counted on the ventral valve; L/W = length/width ratio; T/W = thickness/width ratio.

Ventral umbo strongly curved featuring prominent apical foramen. Strongly anacline interarea that becomes nearly perpendicular to commissural plane towards tip, projecting over hingeline. Delthyrium mostly open, flanked by minute deltidial plates visible on well-preserved specimens. Carinate, with flat-topped ventral fold with medial interspace flanked by a pair of prominent ribs on each side.
Dorsal umbo minute and incurved, obscuring open dorsal interarea. Broad dorsal sulcus usually containing single prominent medial rib flanked by two smaller ribs beginning near umbo with a single rib forming each flank of the sulcus. Medial rib larger than other ribs on shell, but similar in prominence to ribs flanking ventral fold.
Dental plates thin, thickening slightly towards anterior, especially at bases, with small dental cavities infilled with secondary shell material towards posterior. Small teeth pointed dorso-medially with weak medial grooves forming accessory lobes.
Minute myophragm near posterior of dorsal valve. Sockets small with weak medial ridge forming tight interlocking hinge with teeth. Hinge plates fuse to form cruralium, rising off the base of the ventral valve towards anterior and supported by a medial septum. Crural bases flat with crura becoming rod-like towards anterior. Dorsomedially directed spiralia consisting of up to four whorls connected by ventro-anteriorly pointed jugum high in dorsal valve near posterior of spiralia.
Remarks
Zygospira modesta appears to be the most common and long ranging species of Zygospira in the eastern United States. Middle Katian (Maysvillian) specimens of Z. modesta have been widely reported as occurring alongside Z. cincinnatiensis James in Meek, Reference Meek1873, and Z. concentrica Ulrich, Reference Ulrich1879 (the latter species herein synonymized with Z. modesta) in this region while late Katian (Richmondian) specimens occur in strata of similar age to Z. kentuckiensis. The younger late Katian specimens are notably longer than wide in comparison to the middle Katian specimens based on the collections measured herein (Fig. 6).
The late Katian (Richmondian) Z. modesta primarily differ from Z. kentuckiensis based on their much smaller size, a more prominent and angular dorsal sulcus and ventral fold, and fewer ribs on the flanks of the shell than Z. kentuckiensis. Given that ribs generally do not increase in number as the shell grows (other than a few uncommon examples of bifurcation in some specimens), this seems a reliable diagnostic character to differentiate the species.
The earlier species are more problematic to differentiate. Zygospira cincinnatiensis was differentiated from Z. modesta in having fewer ribs on the lateral flanks of the shell (typically five) and generally larger size (Foerste, Reference Foerste1910). It would be difficult to differentiate Z. cincinnatiensis and Z. modesta of similar sizes, however, perhaps indicating that Z. cincinnatiensis is more common than reports would suggest. Zygospira cincinnatiensis is tentatively retained here, but the division between these specimens remains somewhat indistinct.
Zygospira concentrica was differentiated from Z. modesta based on having stronger concentric striae, no radiating plications (ribs), straighter posterior margins, and a more pointed ventral beak (Foerste, Reference Foerste1910). The apparent lack of ribs in specimens of Z. concentrica seems to reflect preservation rather than any real diagnostic difference because examination of collections of species from the Cincinnati region revealed weak ribs that appear to have been abraded in most specimens identified as Z. concentrica. Furthermore, the strong concentric striae probably represent a prominent interruption in the growth of the shell and should not be considered a reliable diagnostic character. Zygospira concentrica is thus herein synonymized with Z. modesta.
An in-depth analysis of the configuration of the spiralia is outside the scope of this study, but it is perhaps notable that the jugum appears slightly closer to the posterior in our sectioned specimen than in the sections illustrated by Copper (Reference Copper1977). It is still located postero-dorsally, however.
Zygospira cincinnatiensis James in Meek, Reference Meek1873
Figure 7
- Reference Hall1847
Atrypa modesta Say in Hall, p. 141, pl. 33, fig. 15c; not pl. 33, fig. 15a, b.
- Reference Meek1873
Zygospira cincinnatiensis James in Meek, p. 126, pl. 11, fig. 5a–c.
- Reference Hall and Clarke1894
Zygospira cincinnatiensis; Hall and Clarke, pl. 54, figs. 13, 14.
- Reference Foerste1910
Zygospira cincinnatiensis; Foerste, p. 30, pl. 6, figs. 16a, b.

Figure 7. Zygospira cincinnatiensis types from the Fairmount Beds (Cincinnatian = Katian) near Cincinnati, Ohio. (1–5) Lectoype FM UC16-a: dorsal, ventral, lateral, anterior, posterior views; (6–10) figured paratype FM UC164-b: dorsal, ventral, lateral, anterior, posterior views. Zygospira cincinnatiensis from the Hudson River Group near Cincinnati, Ohio (from Hall's type collection for Atrypa modesta). (11–15) AMNH 29837, dorsal, ventral, anterior, posterior, and lateral views. Scale bars = 5 mm.
Types
Foerste (Reference Foerste1910) explained that the original types (collected by James and described by Meek, but credited to James by Meek) were not deposited in a known institution and suggested that the collection numbered UC164 from the Fairmount Beds near Cincinnati, Ohio, be regarded as “typical” (Foerste, Reference Foerste1910, p. 30). He reasoned that Meek would have received the original type specimens from James whose collection was then housed at the University of Chicago but is now located at the Field Museum in Chicago. One of the specimens from the original UC164 collection has been selected here as a more typical neotype (FM UC164-a).
A single specimen in the Field Museum collection is marked as a plesiotype (FM UC12391) and was apparently figured by Hall and Clarke (Reference Hall and Clarke1894), but this specimen has far more ribs than is typical for Z. cincinnatiensis. It is unlikely that this specimen was collected from the same locality, although this is not clear from the label associated with the specimen. In museum records, this is noted as belonging to the Hall collection while the other specimens are part of the James collection. This may represent a single, unusual example of Z. cincinnatiensis or could represent a subspecies, but it is difficult to draw any conclusions based on a single shell. Although it has a similar number of ribs to Z. kentuckiensis, it more closely resembles Z. cincinnatiensis in having a more prominent fold and sulcus.
Occurrence
Zygospira cincinnatiensis seems to be restricted to the middle Katian (Maysvillian) in the Cincinnati area, although further detailed study of larger collections of Zygospira may extend its range into the late Katian (Richmondian). Foerste (Reference Foerste1910) mentioned that ancestral forms of the species are found in the Edenian (lower to middle Katian) that have less conspicuous bifurcation of ribs on the fold. Given the doubtful utility of rib bifurcation as a diagnostic feature in this genus, these specimens should likely be considered Z. cincinnatiensis as well.
Remarks
Zygospira cincinnatiensis was distinguished from Z. modesta by Meek based on the presence of bifurcating ribs on the ventral fold, but bifurcating ribs are sometimes present in large collections of Z. modesta as well and may not be a reliable diagnostic character (also noted by Foerste, Reference Foerste1910). The elevated ventral fold that was mentioned, however, is more diagnostic, and Z. cincinnatiensis is, on average, larger than Z. modesta (Fig. 8). Both differ from the similar-sized Z. kentuckiensis in having a significantly more prominent fold with a matching prominent medial rib in the dorsal sulcus. Zygospira cincinnatiensis also has consistently fewer ribs than both Z. modesta and Z. kentuckiensis when large collections are compared.
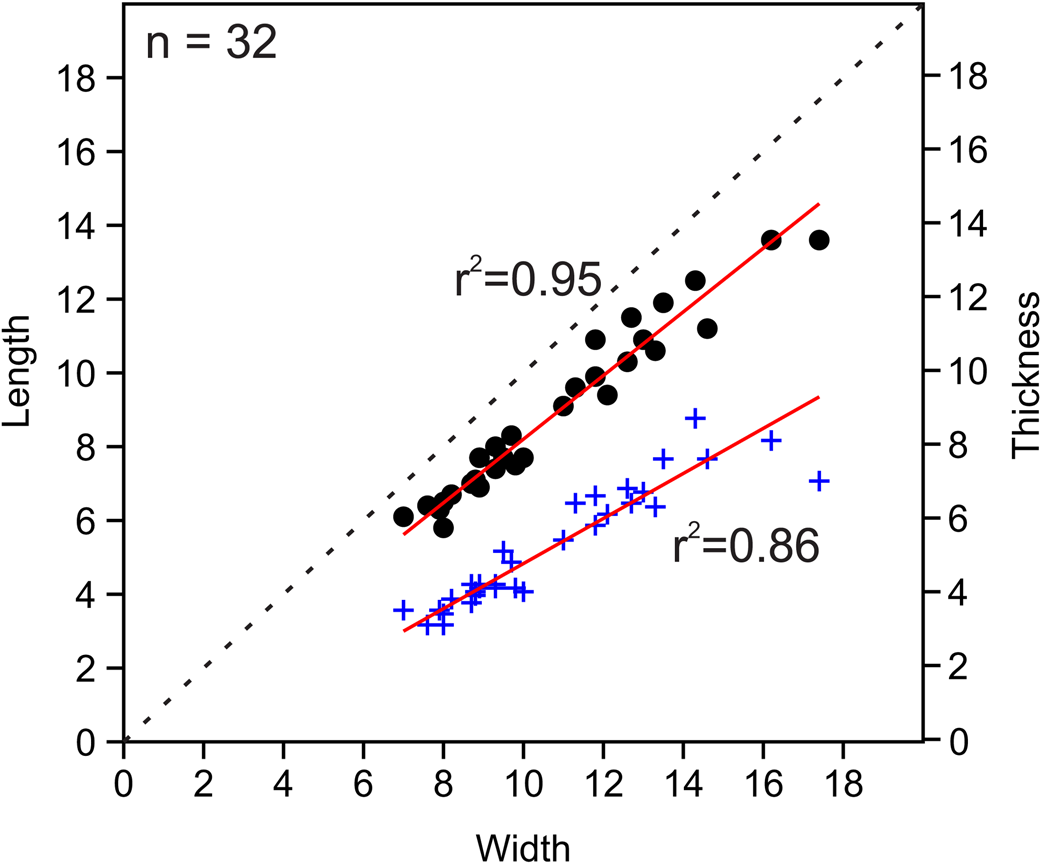
Figure 8. Bivariate plots of length vs. width and thickness vs. width for measured Zygospira cincinnatiensis specimens. Circles indicate length vs. width; plus signs represent thickness vs. width.
It is difficult to determine the precise abundance and stratigraphic range of this species. Although apparently easily differentiated from Z. modesta in isolated collections (especially when the Z. cincinnatiensis are larger), it can be difficult to differentiate these species when specimens of similar sizes are compared. When Z. cincinnatiensis is small, the shells very closely resemble large Z. modesta in every way other than having fewer ribs on average (14–16 in Z. cincinnatiensis rather than 16–18 in Z. modesta). These smaller shells are nearly identical in terms of length, width, and depth to Z. modesta and lack the distinctive raised fold of larger shells (Table 2). Larger shells are easily differentiated from Z. kentuckiensis shells, which have more ribs and much less prominent dorsal sulcus and ventral fold.
Table 2. Summary statistics for Zygospira cincinnatiensis specimens measured for this study (n = 32). L = length; W = width; T = thickness (depth); Ad = deflection at anterior of the commissure (all in mm); Aa = apical angle in degrees; R = number of ribs counted on the ventral valve; L/W = length/ width ratio; T/W = thickness/width ratio.
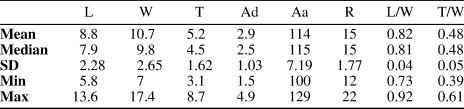
One of the paratypes of Z. modesta from the American Museum of Natural History (AMNH 29837) is herein considered Z. cincinnatiensis. It is unclear whether all the Z. modesta type specimens were collected from the same locality, but if that was the case, Z. modesta co-occurs with Z. cincinnatiensis as the species are currently defined.
Zygospira kentuckiensis James, Reference James1878
Figures 9, 10.1–10.10, 11
- Reference James1878
Zygospira modesta var. kentuckyensis James, p. 7.
- Reference Nettelroth1889
Zygospira kentuckiensis James in Nettelroth, p. 138, pl. 34, figs. 21–25.
- Reference Hall and Clarke1894
Zygospira kentuckiensis; Hall and Clarke, pl. 54, figs. 11, 15, 16.
- Reference Foerste1924
Zygospira kentuckiensis; Foerste, p. 127, pl. 10, fig. 20a–c, pl. 15, figs. 1a–p, 2a, b, 4a–c.

Figure 9. Zygospira kentuckiensis. (1–10) Types from Cincinnatian (= Katian) of Jefferson County, 18 miles east of Louisville, Kentucky; (1–5) neotype FM UC96-a dorsal, ventral, lateral, anterior, posterior views; (6–10) figured paratype FM UC96-b, dorsal, ventral, lateral, anterior, posterior views; (11–15) illustrated plesiotype FM UC 12390 in Hall and Clarke (Reference Hall and Clarke1894) from Cincinnatian (=Katian) of Oldham County, Kentucky, dorsal, ventral, lateral, anterior, posterior views. Scale bars = 5 mm.

Figure 10. (1–10) Zygospira kentuckiensis from the Queenston Shale (late Katian) at Big Bay near Owen Sound, Ontario. (1–5) ROMIP 66852 dorsal, ventral, lateral, anterior, posterior views; (6–10) ROMIP 66853 dorsal, ventral, lateral, anterior, posterior views. (11–15) Zygospira kentuckiensis meafordensis from the Queenston Shale near Meaford, Ontario (GSC 8514); (11) small slab with several shells on surface; (12) dorsal valve attached to piece of rock; (13) exterior of isolated dorsal valve attached to slab in (11); (14) interior of dorsal valve attached to slab in (11); (15) magnified view of (14). Scale bars = 5 mm (1–14); = 1 mm (15).
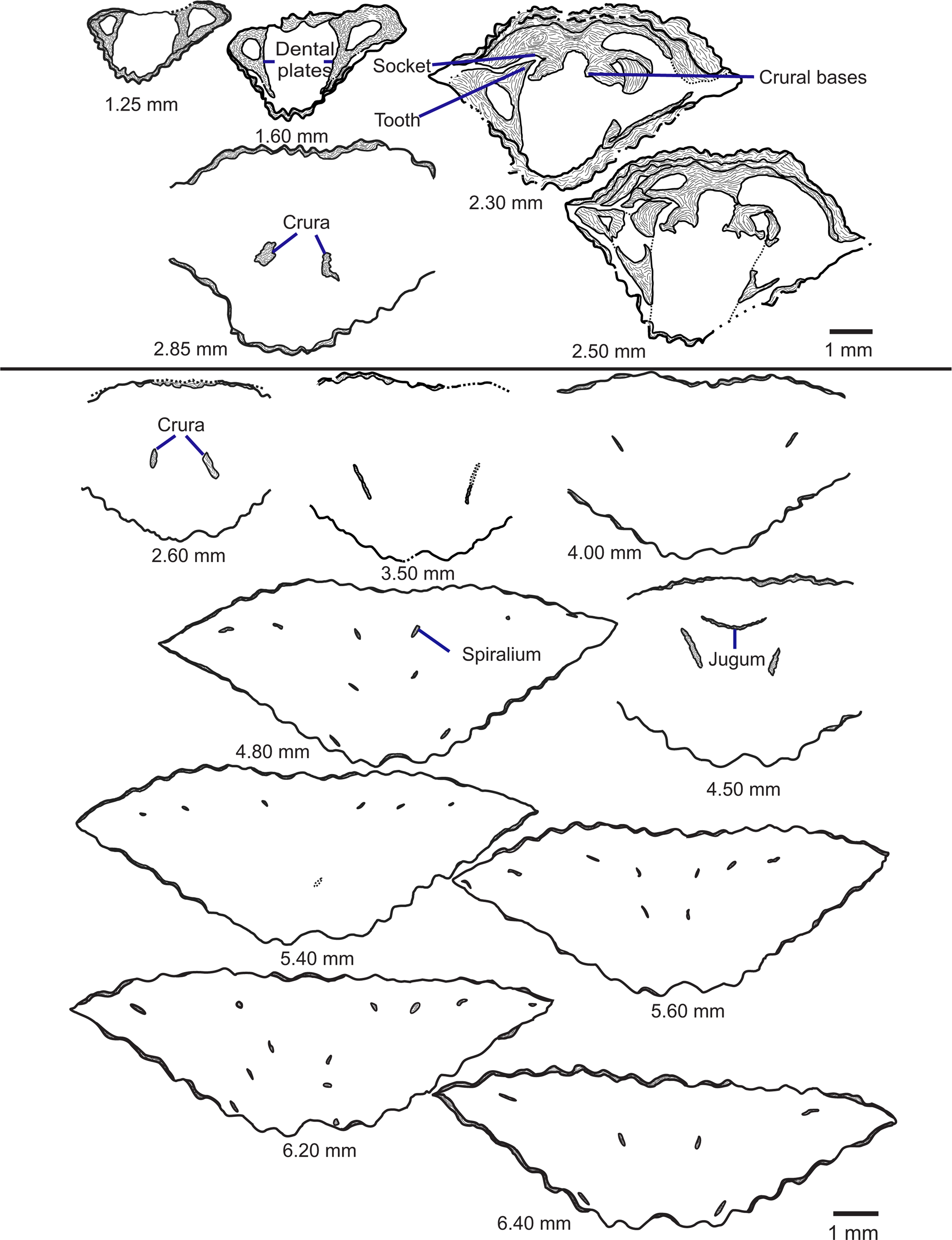
Figure 11. Tracings of serial sections of Zygospira kentuckiensis from the Queenston Formation at Big Bay near Owen Sound, Ontario (ROMIP 66854). Numbers represent distance from posterior of ventral umbo in mm at which the section was ground. Figure represents two sectioned shells to show the complete internal morphology of species (above and below line). Scale bar = 1 mm.
Types
The species was first mentioned by James (Reference James1878) in brief, describing fossils from Nettelroth from the upper part of the Cincinnati Group, Jefferson County, Kentucky, 18 miles east of Louisville. Nettelroth (Reference Nettelroth1889) later described the species in more detail himself, explaining that the species is found in different places in Oldham County, Kentucky, and that it is found in great abundance at Taylor's Station but also on the Shelby Railroad in the shales of the Hudson River Group. Unfortunately, there is no indication of where Nettelroth may have deposited his collection, although a large collection of Nettelroth's fossils was apparently donated to the US National Museum (now Smithsonian Institution) after his death.
A small collection of six shells within James’ collection at the Field Museum labeled UC96 apparently were collected from the exact locality mentioned in James’ (Reference James1878) initial description of the species (Jefferson County, 18 miles east of Louisville, Kentucky) and is thus likely the original type collection. A single specimen from UC96 (FM UC96-a) is herein selected as a lectotype for Z. kentuckiensis.
Another collection of Z. kentuckiensis from the Field Museum labeled IP 12390 consists of a single well-preserved and carefully cleaned specimen. This shell apparently was illustrated by Hall and Clarke (Reference Hall and Clarke1894), but the specimens from the James collection (FM UC96) have priority as types.
Diagnosis
Shells large for the genus, usually wider than long, and ventribiconvex in profile; simple ribs numbering >18 but less prominent than other species; relatively weak ventral fold with medial interspace accentuated by two ribs on each flank and broad and shallow dorsal sulcus with weak medial ridge; thick dental plates and hinge plates for the genus with simple dorso-posteriorly located jugum; prominent teeth with hook-like transverse profile towards anterior.
Occurrence
Upper Katian (Richmondian) of eastern North America. Common in the upper Fairmount (and correlated units) in the Cincinnati area and the Queenston Shale in Ontario.
Description
Shell medium-sized, with a mean length of 9.8 mm, width of 10.9 mm, and a thickness of 5.5 mm (Table 3); outline subpentagonal with outline wider than long (mean of 91% as long as wide) and ventribiconvex in lateral profile with ventral valve ~1.5 times as deep as dorsal valve. Astrophic hingeline with rounded lateral flanks. Anterior margin unisulcate forming prominent tongue. Ribs strong and rounded, simple with rare bifurcations and becoming larger towards anterior, typically numbering from 18–22 but can be more numerous (up to 28 in collections examined herein). Fine growth lamellae visible especially near anterior margin on well-preserved shells.
Table 3. Summary statistics for Zygospira kentuckiensis specimens measured for this study (n = 110). L = length; W = width; T = thickness (depth); Ad = deflection at anterior of the commissure (all in mm); Aa = apical angle in degrees; R = number of ribs counted on the ventral valve; L/W = length/ width ratio; T/W = thickness/width ratio.

Ventral umbo strongly curved, featuring prominent apical foramen. Strongly anacline interarea that becomes nearly perpendicular to commissural plane towards tip, projecting over hingeline. Delthyrium open, flanked by minute deltidial plates visible on some well-preserved specimens. Carinate, with less prominent flat-topped ventral fold with medial interspace flanked by a pair of prominent ribs on each side beginning near umbo.
Dorsal umbo minute and incurved, obscuring dorsal interarea. Broad, weak dorsal sulcus containing single prominent medial rib flanked by two smaller ribs beginning near umbo with no obvious prominent flanking ribs. Medial rib generally most prominent.
Dental plates moderately thick, divergent near posterior of ventral valve and convergent and thinner towards anterior. Dental cavities small. Teeth prominent, thickened, and curved, becoming increasingly curved towards anterior with variable weak accessory lobes separated from main lobe by weak medial groove. Ventral muscle scars poorly impressed but serial sections show evidence of small divergent adductor scars separated by slightly raised diductor platform.
Minute myophragm variably present near posterior of dorsal valve. Sockets curved with weak medial ridges, forming tight interlocking fit with teeth in dorsal valve. Median septum forms cruralium with hinge plates. Crural bases flat but crura become rod-like and laterally directed towards the anterior. Spiralia with dorso-medially directed apices consist of up to five whorls. Jugum located high in dorsal valve posterior to spiralia and curved towards anterior.
Remarks
Zygospira kentuckiensis is similar in shape to the type species Z. modesta, but Z. kentuckiensis is significantly larger (Fig. 12) and has more numerous ribs (18–22 in Z. kentuckiensis in comparison to the 16–18 in Z. modesta). The two ribs that flank each side of the ventral medial fold are less prominent in this species in comparison to Z. modesta and form a shallower medial interspace on the ventral valve. The corresponding medial rib in the dorsal sulcus is also less prominent in Z. kentuckiensis.
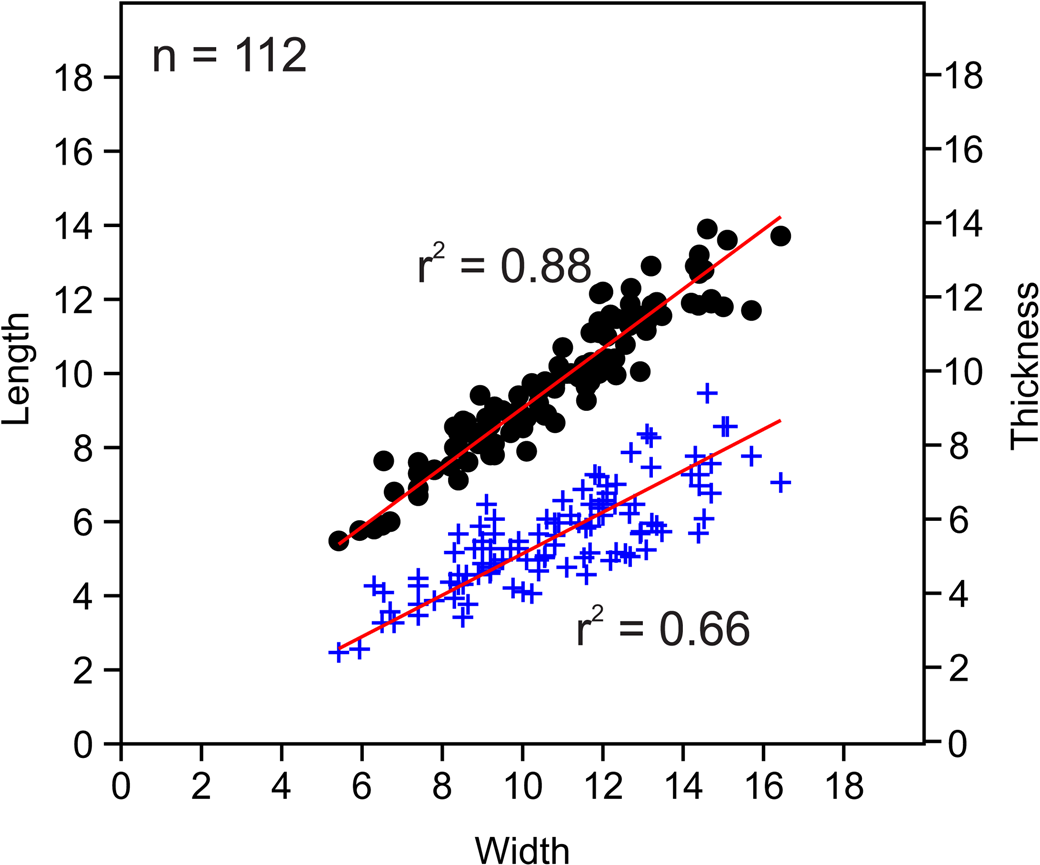
Figure 12. Bivariate plots of length vs. width and thickness vs. width for measure Zygospira kentuckiensis specimens. Circles indicate length vs. width; plus signs represent thickness vs. width.
There are slight differences in the interior of the shell as well. Zygospira kentuckiensis has significantly thicker dental plates and hinge structures in comparison to Z. modesta, as well as a more thickened posterior shell. This thickening may simply reflect the growth of a larger shell in the case of Z. kentuckiensis, but Z. modesta appears to have consistently thinner shells (based on the 5 specimens of each species sectioned here).
New material (Figs. 10.1–10.10, 11) was collected from the Queenston Formation at Big Bay near Owen Sound, Ontario (44.796537°N, 80.924807°W). These shells are similar to typical shells from the Cincinnati area but are slightly less convex. Zygospira kentuckiensis shells are common and numerous at this locality, forming low-diversity shell beds that form packstones comprised of this one species. Some of the exposed bedding planes show wave ripples, implying that Zygospira may have been able to tolerate relatively high energy environments above fair-weather wave base.
Zygospira richmondensis Caley, Reference Caley, Wilson, Caley, Sproule and Okulitch1936, which was described from the Kagawong Formation (Richmondian, latest Katian) on Manitoulin Island, is represented by a single shell in the collections of the Royal Ontario Museum in Toronto (ROMIP 12448). This specimen resembles Z. kentuckiensis in size but seems to have a shallower fold and sulcus than is typical from the species. Given that all other characteristics are similar, however, it is here synonymized with Z. kentuckiensis.
Zygospira kentuckiensis meafordensis (Foerste, Reference Foerste1924)
Figure 10.11–10.14
- Reference Foerste1924
Zygospira meafordensis Foerste, p. 128, pl. 15, figs. 3a–c.
Types
Lectotype GSC 8514A selected from GSC 8514 collected from Concession VIII, lot 24, ~4 miles northwest of Meaford (Foerste, Reference Foerste1924). Although these shells are illustrated as isolated shells, all three illustrated specimens are embedded in matrix. GSC 8514A is a ventral valve, GSC 8514B is a dorsal interior, and GSC 8514C is wider than a typical ventral valve.
Occurrence
Specimens in the GSC collection were collected from only a single locality near Meaford, Ontario, from the Queenston Formation, which was described by Foerste (Reference Foerste1924) as being at Concession VIII, lot 24, 4 miles northwest of Meaford. Two other localities are noted from the same region.
Remarks
This species very closely resembles Z. kentuckiensis differing only in the much more numerous ribs (26–30 in this subspecies versus 18–22 in Z. kentuckiensis) and in its slightly smaller size, based on the limited number of specimens available for study. The fold and sulcus are broad and shallow, as is typical of Z. kentuckiensis, but Foerste noted that the slope between the fold and sulcus and the shell flanks is more gradual in these specimens in comparison to Z. kentuckiensis. Foerste (Reference Foerste1924) described a cardinal process, but no cardinal process is visible in the type material (GSC 8514, Fig. 10). Given the extremely limited range of this species and overall similarity in shell size and shape, Z. meafordensis is herein regarded as a s subspecies of Zygospira kentuckiensis with which it is found in Ontario.
Zygospira resupinata Wang, Reference Wang1949
Figure 13
- Reference Wang1949
Zygospira resupinata Wang, p. 18, pl. 10, fig. A (1–12).

Figure 13. (1–10) Zygospira resupinata types from Brainard Shale, Maquoketa Formation in Jackson County, Iowa. (1–5) Holotype SUI 1874, dorsal, ventral, lateral, anterior, posterior views; (6–10) paratype SUI 1873, dorsal, ventral, lateral, anterior, posterior views. Scale bars = 5 mm.
Types
SUI 1874 (holotype), SUI 1873 (paratype) from the Cornulites zone of the Brainard member; south corner of section 29, Fairfield township, Jackson County, Iowa (Wang, Reference Wang1949).
Occurrence
Upper Katian (Richmondian) of central North America.
Description
See Wang (Reference Wang1949).
Remarks
Wang only deposited two specimens in the State University of Iowa collection. Based on these two specimens (Table 4) and his descriptions, this species differs from all other species of Zygospira in being longer than wide and having far fewer costae (12 versus the typical 18–22 of Z. modesta). It also differs from other species in having a smaller and narrower ventral fold and dorsal sulcus, creating a less prominent deflection of the commissure near the anterior. The interior of this species remains unknown, but the external characteristics suggest affinities with Zygospira, although this species shares the more elongate shell shape and narrower fold and sulcus of the earlier Anazyga. A single damaged Zygospira resupinata multicostata Howe, Reference Howe1965, shell from the Hudson Bay Lowlands (Jin et al., Reference Jin, Caldwell and Norford1997, pl. 30, fig. 21) exhibits typical Zygospira-style spiralia, but this has not been described in the material from Iowa. If this feature is confirmed in Zygospira resupinata sensu stricto, the species may represent an earlier divergence from earlier atrypide stocks (such as Anazyga), although the lack of early occurrences of this species at a time when species such as Z. modesta were becoming widespread makes this unlikely.
Table 4. Measurements of Zygospira resupinata types. L = length; W = width; T = thickness (depth); Ad = deflection at anterior of the commissure (all in mm); Aa = apical angle in degrees; R = number of ribs counted on the ventral valve; L/W = length/ width ratio; T/W = thickness/width ratio.

Large collections of this species are currently unavailable for study, but Z. resupinata and Z. resupinata multicostata Howe, Reference Howe1965, from the Montoya Group in Texas and the Surprise Creek and Caution Creek formations in the Hudson Bay Lowlands represent the farthest that Zygospira was able to infiltrate into the inland seas of Laurentia. No atrypides are yet known from the paleoequatorial seas of southern Manitoba despite a relatively diverse brachiopod fauna being reported there (Jin and Zhan, Reference Jin and Zhan2001).
Results
Two principal component analysis scattergrams were produced to illustrate the differences in morphology as represented shell measurements between Zygospira species in eastern North America (Figs. 14, 15). Specimens were primarily grouped by species (color) and region (symbols).

Figure 14. Principal component analysis (PCA) scattergram showing PC1 plotted against PC2. Loadings for PC1 (bottom left) and PC2 (bottom center) shown along with scree plot (bottom right). Blue = Z. modesta from localities near Cincinnati, Ohio (dots = specimens from the middle Katian, plus signs = specimens from upper Katian); green = Z. kentuckiensis (dots = specimens from the Cincinnati region, plus signs = specimens from Ontario); black = Z. cincinnatiensis from area near Cincinnati; red = Z. resupinata types from upper Katian of Iowa; gray = Z. concentrica from area near Cincinnati.

Figure 15. Principal component analysis (PCA) scattergram showing PC2 plotted against PC3. Loadings for PC2 (bottom left) and PC3 (bottom center) shown along with scree plot (bottom right). Blue = Z. modesta from localities near Cincinnati, Ohio (dots = specimens from the middle Katian, plus signs = specimens from upper Katian); green = Z. kentuckiensis (dots = specimens from the Cincinnati region, plus signs = specimens from Ontario); black = Z. cincinnatiensis from area near Cincinnati; red = Z. resupinata types from upper Katian of Iowa; gray = Z. concentrica from area near Cincinnati.
Two groups of species can be recognized in the PC1 (principal component one) versus PC2 scattergram (Fig. 14): one comprised primarily of Z. modesta shells and the other representing both Z. kentuckiensis and Z. cincinnatiensis. The two type specimens of Z. resupinata plot within the Z. modesta cluster, but given the small sample size here, the significance of this is debatable. The shells of Z. concentrica plot between these two main groups, although much higher on the PC2 axis. Zygospira modesta and Z. kentuckiensis overlap considerably, reflecting broad similarities in their shell morphology.
The biplots at the center of the axes provide some indication of the covariance of the measurements and proxies for shape. Most of the linear measurement vectors (length, width, thickness) approximately align with the horizontal axis (PC1) while the proxies for shape (ratios of measurements) align with the vertical axis (PC2). This indicates a strong size influence on PC1 while PC2 approximately represents most of the variance in shape within the dataset. These relationships also are clearly illustrated in the loadings plots below the figure for PC1 (bottom left) and PC2 (bottom center). The scree plot (Fig. 14, bottom right) illustrates that most of the variance is captured by PC1 (52%) while PC2 represents 15% of the variance.
Some intraspecific morphological variation is revealed in the Z. modesta cluster. Younger specimens plot higher on axis 2 (Fig. 13) than older specimens. This is mainly due to a more elliptical shell (smaller length/width value), slightly larger apical angle, and slightly less prominent anterior fold and sulcus (anterior deflection/width) in the older, primarily Maysvillian shells in comparison to the younger Richmondian sample.
A second PCA scattergram (Fig. 15) plots PC2 and PC3. This shows a different pattern, with more-significant overlap between the groups represented by species. As in the previous figure, PC2 is positively correlated with the proxies for shape in the dataset while PC3 is influenced mainly by the number of ribs. The significant overlap between species in the scattergram reflects that size (represented mostly in PC1 rather than PC2 or PC3) is the most diagnostic character separating the species while shell shape is broadly similar among species of Zygospira. There is more significant interspecific variation in the number of ribs in this plot, however. PC3 represents a relatively small proportion of the variance, but the effects of shell size and shape are represented by a greater number of variables, and thus are perhaps over-represented in the variance of the dataset.
Note that in both cases, variables were standardized using a correlation matrix within PAST before plotting the scattergrams. This ensures that the variance in the dataset is more reasonably represented rather than working with the large ranges in values within the raw dataset. It also ensures that variables with large absolute ranges do not dominate the variance. In this study, the relatively large values for apical angle and ribs would overprint any trend in small values such as the depth of the sulcus.
Discriminant analysis (DA) also was run on the dataset to test whether the existing classification scheme can adequately discriminate between the species as they are currently defined, producing a scattergram that represents the maximum variance between samples (Fig. 16). Most of the data points are near zero on axis 1, with the Z. concentrica samples clustering together far to the left (top). This supports the hypothesis that Z. concentrica should be synonymized and likely represents deformed and abraded shells of Z. modesta, given that they plot so far out of the normal morphological range of Zygospira.

Figure 16. Discriminant analysis (DA) scattergram showing the morphological difference between all species (top) and all species excluding Z. concentrica (bottom). Blue = Z. modesta from localities near Cincinnati, Ohio (dots = specimens from the middle Katian, plus signs = specimens from upper Katian); green = Z. kentuckiensis (dots = specimens from the Cincinnati region, plus signs = specimens from Ontario); black = Z. cincinnatiensis from area near Cincinnati; red = Z. resupinata types from upper Katian of Iowa; gray = Z. concentrica from area near Cincinnati.
When Z. concentrica is excluded, the specimens form a more even distribution across the scattergram with very little overlap between clusters (Fig. 16, bottom). The confusion matrix produced by the analysis shows relatively stable classifications (Tables 5, 6), seemingly indicating that size is a good diagnostic character to differentiate species as they are currently defined. Whether size should be considered a diagnostic character requires some further consideration.
Table 5. Confusion matrix for Zygospira species in Figure 15 (top). Rows are given groups while columns are predicted groups. Note that 93.4% of specimens were correctly identified.
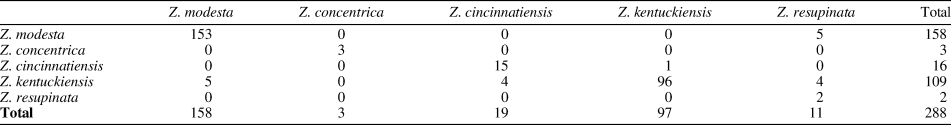
Table 6. Confusion matrix for Zygospira species in Figure 15 without Z. concentrica (bottom). Rows are given groups while columns are predicted groups. Note that 93.31% of specimens were correctly identified.

Discussion
Speciation or ontogeny?
If species have similar shapes but different sizes, perhaps they represent different stages of growth (ontogeny) rather than different trajectories of evolution (speciation). The two most common species of Zygospira are found in rocks of the same age in the late Katian (Richmondian) with only minor differences in the shell shape (as shown by slightly different L/W and T/W values for Z. modesta and Z. kentuckiensis; Tables 2, 3, see also Howe, Reference Howe1965, text-fig. 4). These differences are much less significant than the difference between the eastern species and the western species, such as Z. resupinata from Iowa and the subspecies Z. resupinata multicostata from Texas and the Hudson Bay Lowlands.
There are morphological differences between these species that are more difficult to explain through ontogeny, however. The number of ribs differ between these two species (mean of 17 on Z. modesta specimens compared to 21 on Z. kentuckiensis). Although it is not unusual for a larger brachiopod shell to have more numerous ribs, either through bifurcation of ribs towards the anterior or insertion of additional ribs in interspaces through intercalation as the shell grows, Zygospira show little to no multiplication of ribs as shells become larger within species (except for some populations of Z. resupinata multicostata—see Jin et al., Reference Jin, Caldwell and Norford1997, pl. 30). If Z. kentuckiensis were simply a form of Z. modesta that was able to biomineralize a larger shell in a more favorable environment, for example, Z. kentuckiensis should have larger ribs rather than more numerous ribs based on the limited variability in the number of ribs within a species, regardless of size.
Furthermore, the shapes of the ventral fold and dorsal sulcus differ considerably between smaller and larger species, with Z. modesta exhibiting a distinctive raised fold and depressed sulcus in comparison to the much less prominent fold and sulcus of Z. kentuckiensis. This significant change in shell shape, which would be difficult to explain as an effect of shell growth, is more reasonably explained as interspecific variation. The larger Z. cincinnatiensis measured herein maintain their more distinctive fold and sulcus in larger shells, similar to the smaller Z. modesta, indicating that this feature is a useful diagnostic tool, although it is difficult to recognize in deformed shells. The profile of the fold and sulcus is known to vary with shell growth among species of other atrypide lineages (e.g., Thulatrypa and Meifodia from the early Silurian—see Huang et al., Reference Huang, Baarli, Zhan and Rong2016; Baarli, Reference Baarli2022).
There are also subtle differences in the size and shape of the internal characteristics of Z. modesta and Z. kentuckiensis. The larger, more robust dental plates of Z. kentuckiensis could be a result of shell thickening with growth of the animal, but this pattern of growth is known in some early Silurian pentameride lineages that are thought to have increased the thickness of the posterior of their shells to stabilize their living position (Ziegler et al., Reference Ziegler, Boucot and Sheldon1966). This may have been an adaptation in the larger Z. kentuckiensis to stabilize their shell in position the seafloor with the commissure oriented upward. The shape of the jugum associated with the spiralia differs between these species as well (Figs. 5, 11), but given how fragile this structure is, this may be a taphonomical artefact rather than a real morphological difference. Further study of the internal morphology of the brachidia in these and other early atrypide species is needed to determine the degree of intra- and interspecific variation of this structure in early members of the lineage.
Besides morphological differences that are difficult to reconcile, the geographic range of species differ. Zygospira modesta is almost entirely restricted to the area around Cincinnati throughout its stratigraphic range, while Z. kentuckiensis has a wider distribution across eastern North America during the late Katian (Richmondian), extending on to the Trenton Shelf in Ontario. If Z. modesta truly were an earlier ontogenetic stage of Z. kentuckiensis, then younger, smaller shells should be found across the same geographic range as the larger shells where both occur in the same stratigraphic interval. Even in the Cincinnati region where both species are known to occur, they are never found in the same beds or at the same locality despite both species being widespread throughout upper Katian (Richmondian) strata in the area, which perhaps indicates that the two species were adapted to different environmental conditions.
The external morphology, internal morphology, and differences in the geographic range of these shells seem to suggest that these shells do, in fact, represent different species. It is relatively uncommon to have multiple fossil brachiopod species of the same genus in the same area, so atypical processes could have driven speciation in Zygospira.
Niche partitioning driving evolution
A variety of processes can contribute to the evolution of new species, but allopatric speciation where populations become isolated by a barrier causing the populations to evolve along different trajectories (Fig. 17) is thought to have produced most species in the fossil record (Eldredge, Reference Eldredge1971; Johnson, Reference Johnson1975) because of the divergence of evolution caused by major environmental shifts in a region (e.g., sea level change, tectonic activity) that would isolate populations of species from one another eventually leading to speciation. This can happen either through vicariance (i.e., a barrier separates two groups within their range) or dispersal (i.e., when a subgroup of a species moves into a new geographic area and subsequently becomes geographically isolated) (Stigall, Reference Stigall2013; Stigall et al., Reference Stigall, Bauer, Lam and Wright2017). Zygospira resupinata and Z. resupinata multicostata may have evolved through dispersal, spreading to the midcontinent region during a time of lower sea level before becoming isolated from the Cincinnati region by the Sebree Trough (Kolata et al., Reference Kolata, Huff and Bergström2001).
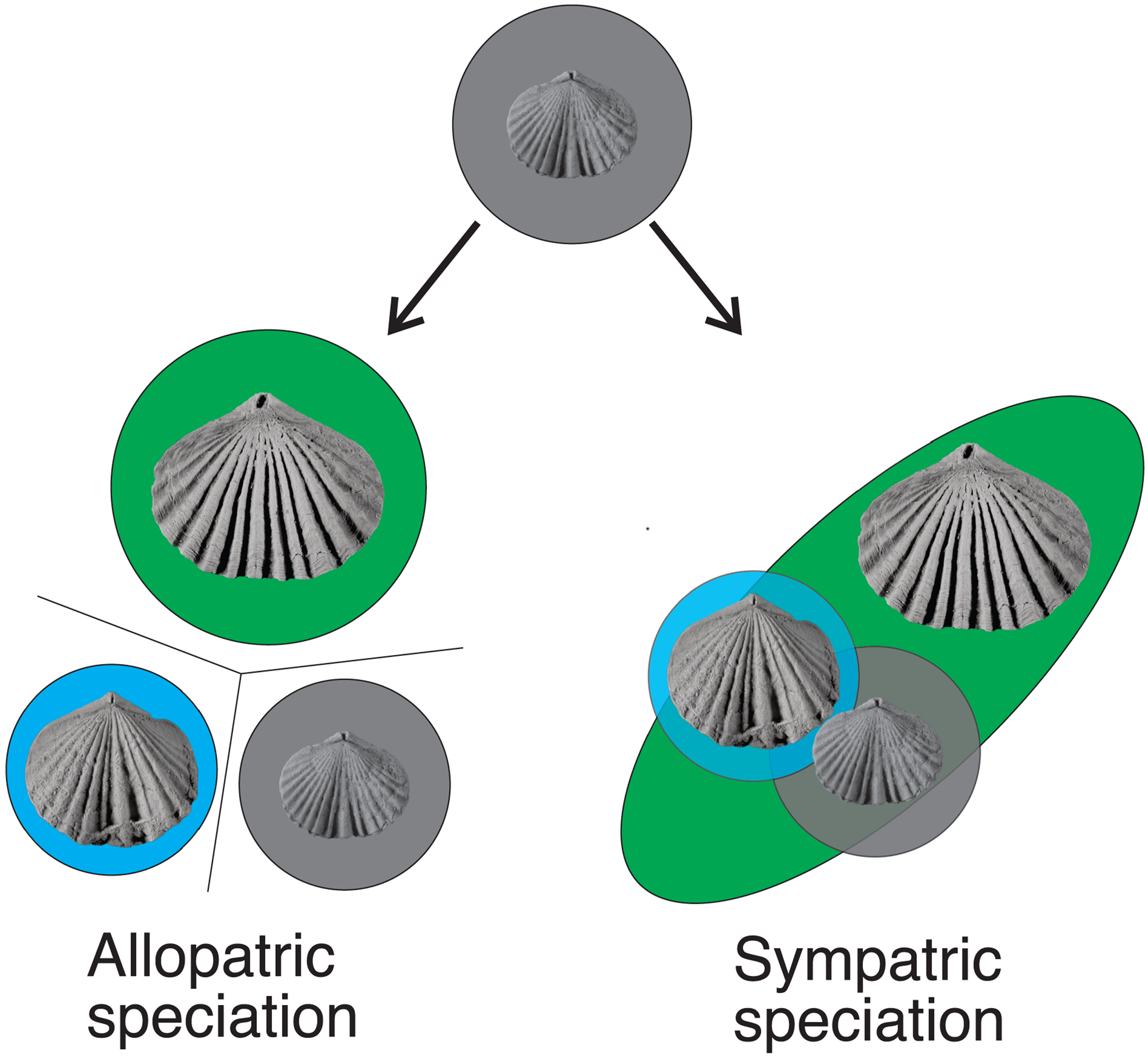
Figure 17. Allopatric and sympatric speciation. Species represented by different background colors (gray = Z. modesta; blue = Z. cincinnatiensis; green = Z. kentuckiensis). The late Katian species of Zygospira in eastern North America likely arose through sympatric speciation, although it isn't yet clear from which lineage Z. resupinata in the interior of North America arose.
The lack of apparent geographic barriers between most Zygospira species in the Cincinnati region rules out allopatric speciation. Other types of speciation, such as peripatric and parapatric speciation, are rarely reported in the fossil record due to the complexities of interpreting niche partitioning in the fossil record, although these processes likely played important roles in planktonic groups (e.g., Lazarus, Reference Lazarus1983; Wei, Reference Wei1994; Jackson and Cheetham, Reference Jackson and Cheetham1999). The evolution of late Katian species of Zygospira may be an uncommon example of sympatric speciation in the fossil record where a single ancestral species gives rise to new species within the same geographic range commonly through the exploitation of slightly different niches or sexual selection that then drives evolution along two different trajectories. Factors influencing sympatric speciation are often difficult to determine in modern ecosystems and are particularly problematic to interpret based on the fossil record where only a small part of the ecosystem is ever preserved.
The larger size of Z. kentuckiensis could have been an advantage in competing with other filter feeders for space on the seafloor where the posterior weighting of the shell, keeping the commissure upright, would have provided an advantage in dense shell beds. Zygospira sometimes occurs in dense accumulations, although whether these are always in situ or reflect taphonomic processes that form condensed beds is not always clear. Other factors could have favored the establishment of one species over the other (e.g., seasonal shifts in nutrient supply, changes in sediment supply, or even changes in the marine ecosystem such as predator-prey cycling), which could have affected whether larger or smaller shells were more successful. The effects of these factors on a single brachiopod lineages from one geographic area are difficult to quantify based on fossil material and would require a more comprehensive study at a regional scale to be meaningfully investigated.
The smaller size of species such as Z. modesta may have been a beneficial adaptation under certain circumstances as well. Shells are commonly found in dense shell beds associated with other filter feeders such as bryozoans, sponges, and crinoids. Often these are only loose associations, but there are examples of Zygospira preserved in place attached to other filter feeders (Fig. 3; see also Sandy, Reference Sandy1996, for an example of Zygospira attached to articulated crinoid columnals). These shells are oriented with the pedicle opening facing the host filter feeders, so these associations are unlikely to be the result of brachiopods randomly settling on the filter feeder after their death. Only the smaller Z. modesta specimens have been preserved attached in place, however. Their smaller size may have been an advantage in comparison to the larger Z. kentuckiensis, if they were living as epibionts on other filter feeders. These attachments would have enabled the brachiopods to feed at a much higher level in the water column than would otherwise be possible, thus avoiding competition for space and food with other brachiopods and filter feeders closer to the seafloor below. This could explain why Z. modesta was able to thrive through the late Katian when many of the other smaller brachiopod species in other lineages mostly disappeared as larger species took over across Laurentia (e.g., as in orthide and rhynchonellide lineages: Sohrabi and Jin, Reference Sohrabi and Jin2013; Sproat and Jin, Reference Sproat and Jin2013; Sproat et al., Reference Sproat, Sohrabi and Jin2014).
Epibiotic life strategies have been exploited by other brachiopod lineages. Carboniferous chonetids are thought to have used their posterior spines to attach to crinoid stems (e.g., Grant, Reference Grant1963). Other lineages, such as the craniiformean brachiopods, are commonly found cemented to filter feeders (including other brachiopods) and were already widespread by the Ordovician (e.g., Chen and Rong, Reference Chen and Rong2019; Bruthansová and Van Iten, Reference Bruthansová and Van Iten2020), although these brachiopods are most commonly cemented to flat surfaces. Most rhynchonelliform brachiopods are not widely known as epibiota, but this life strategy may have been more common than the fossils would suggest given how unlikely this arrangement is to survive taphonomic processes and be preserved in the fossil record.
These types of associations may have played important roles in the evolution of shelly benthos through the Ordovician Radiation and the greater radiation of filter-feeding benthos that occurred through the early Paleozoic. Although the increase in biodiversity associated with this event and some of the contributing extrinsic environmental factors are becoming increasingly clear, we are only beginning to understand some of the intrinsic factors driving radiations (Stigall et al., Reference Stigall, Edwards, Freeman and Rasmussen2019, and references therein). We know particularly little about the complex interplay between filter feeders that led to the opening of new niches and evolution of a wide variety of body plans (although see Vinn and Wilson, Reference Vinn and Wilson2015). Brachiopods, which were among the most common components of the benthic ecosystem at this time, must have played a key role in the evolution of these relationships, but further study is needed to clarify how they may have affected the evolution of other elements of the benthic ecosystem at this time.
Evolution of larger shells with increased sediment influx
Few studies have confidently linked changes in brachiopod shell morphology to environmental factors, but Laurin and García-Joral (Reference Laurin and García-Joral1990) concluded that an increase in clay sedimentation in the Jurassic of the Iberian Range in Spain caused miniaturization of rhynchonellide shells (i.e., evolution of smaller, mature shells). They proposed that these smaller shells were better adapted to an r-mode life strategy (rapid development and maturation to overcome high juvenile mortality), which would have allowed the brachiopods to colonize a difficult living environment quickly. Larger shells would otherwise be an advantage in competing for space in a more favorable living environment where the larger size would be beneficial in gathering food and nutrients from the water column more quickly than smaller shells and could have excluded smaller shells from becoming established.
The carbonate platforms and basins of eastern North America (e.g., the Trenton Shelf across southeastern Canada and New York) were subjected to an increase in clastic sedimentation during the Late Ordovician from the nearby Taconic Mountains, which were uplifted and eroding by this time (Waldron and van Staal, Reference Waldron and van Staal2001; van Staal et al., Reference van Staal, Whalen, McNicoll, Pehrsson, Lissenberg, Zagorevski, van Breemen, Jenner, Hatcher, Carlson, McBride and Martínez Catalán2007; Ganis and Wise, Reference Ganis and Wise2008). Rather than miniaturization, however, larger species of Zygospira evolved and become more widespread than the earlier smaller species. Detailed sedimentological study of all collection localities has not been attempted here, but in general terms, sediments on the St. Lawrence Platform in Ontario become notably argillaceous in the middle to late Katian, especially in the Queenston Formation (Armstrong et al., Reference Armstrong and Carter2010) where Z. kentuckiensis is found in thick successions of dense shells beds. An influx of clay into the region does not seem to have excluded the larger Zygospira, but rather seems to have favored their establishment. Perhaps a larger shell was less likely to have been buried by clastic sediment entering the basin, but further morphometric and paleoecological studies on this and other brachiopod lineages in different regions across the geological time scale are needed to determine whether this is a consistent ecophenotypic trend.
Variation in the spiralia
From the interior of the five shells of Z. modesta and five shells of Z. kentuckiensis examined here, it appears that there is very little intraspecific variation in the structure of the brachidia of Zygospira. Both large and small shells have similar spiralia with almost identical shapes and numbers of whorls. The only notable differences are the shape of the jugum, but the position of the structure is relatively stable and not as variable as once thought (Hall and Clarke, Reference Hall and Clarke1894), the posterior thickening in the larger Z. kentuckiensis, and thicker, more hook-shaped teeth in Z. kentuckiensis. The internal morphology of other species, especially those now assigned to Anazyga, remains to be studied.
The shape and size of the calcified brachidial support structures (spiralia) in Zygospira and the other Anazygidae could reveal the evolutionary affinities of this group to other early atrypides, however. Unfortunately, it is currently difficult to study the internal morphology of large samples of shells unless a researcher comes upon a damaged shell that reveals the spiralia intact. The Atrypidae (and the Rhynchonellidae, along with the other spire-bearing brachiopod lineages) are only rarely found disarticulated due to their tight-fitting cyrtomatodont hinge that holds the shells together even after death, and even then the spiralia is almost always missing. To study the internal morphology of atrypides, researchers must serial section shells—a destructive process that is slow and laborious and requires the use of a parallel grinder, which has not been manufactured in years (although see Zhang et al., Reference Zhang, Sproat, Zhan, Zhang, Luan and Huang2019, for open-source design diagrams to create new machines). Sectioning a single atrypide shell may take several days to capture the entire spiralia and associated structures. The destructive nature of the process makes it difficult to examine historical collections at museums and in other collections where destructive techniques are usually prohibited. Contemporary computed tomography (CT) methods allow for more rapid and less destructive 3-D imaging of brachiopod shells, but have had little success thus far in imaging calcite marine invertebrates preserved in limestones because of the minimal contrast that is common between shell material and surrounding sediment. These techniques hold promise in allowing paleontologists to rapidly image the interiors of a large number of shells to efficiently study variation in structures such as the spiralia and better understand the degree of variation in these structures in early atrypides.
Although not the focus of this study, the shape of the jugum could have some diagnostic utility given the apparent differences between species. The function of this distinctive section of the brachidium that connected the spiralia remains unknown, perhaps playing a role in supporting the two spires of the spiralia that it connects (Rudwick, Reference Rudwick1970) or providing support for the mouth of the brachiopod in life, providing a balance to keep the spiralia level, or playing a role in raising or lowering the spiralia as the brachiopod opened and closed its shell (Copper, Reference Copper1967). Regardless of its function, the jugum may have some utility in the taxonomy of the spire-bearing brachiopods given the conservative nature of the evolution of internal shell structures, but it has been poorly studied in many spire-bearing lineages. Despite this variation in shape of the jugum noted here in Zygospira, there is no indication of the variation in position of the jugum noted by previous workers (e.g., Hall and Clarke, Reference Hall and Clarke1894). This apparent variation was likely a result of lumping both Anazyga and Zygospira under a single genus because a lower position for the jugum near the anterior of the shell is more characteristic of Anazyga (Copper, Reference Copper1977). Again, the significance of these differences remains uncertain until a better understanding of the jugum has been developed.
Conclusions
The early atrypide brachiopod Zygospira spread across much of Laurentia during the late Katian. Zygospira species are difficult to differentiate because of the lack of typical diagnostic features useful in other brachiopod lineages, but they differ from one another mostly based on size and the number of ribs on the shell rather than by shape. Although this may indicate that species simply represent different stages in growth or growing conditions, this does not seem to be the case. Although the large Z. kentuckiensis do consistently possess more ribs, large examples of Z. cincinnatiensis show fewer ribs despite reaching similar shell sizes. Key differences between species in the shape of the ventral fold and dorsal sulcus and structures in the interior such as the jugum would be difficult to produce through ontogeny.
Despite the difficulty in classifying species, the taxonomy of the genus can be refined although the key to classifying these species may rely on a more detailed examination of the morphology of the spiralia and its associated structures. Zygospira concentrica can be synonymized with Z. modesta because the concentric growth lamellae are more likely to represent local environmental disturbances and are unlikely to be diagnostic. The newly reported specimens from the area near Owen Sound, Ontario are typical Z. kentuckiensis that differ only in their slightly less convex shells from the type specimens from the Cincinnati area. Zygospira meafordensis is herein considered a subspecies of Z. kentuckiensis based on its similar morphology and its extremely limited distribution.
While many other brachiopod lineages seem to have evolved in a stepwise fashion through the Late Ordovician with older, smaller species being replaced by younger, larger, and more globose species, the older and smaller Zygospira modesta co-existed with younger species that evolved throughout the Katian (e.g., Z. cincinnatiensis, Z. kentuckiensis, and Z. resupinata). Although the species vary significantly in size, their shell shapes are notably similar, except for Z. resupinata, which differs in ornamentation, shell shape, and shape of the dorsal sulcus and ventral fold. That several species evolved in the Cincinnati area at the same time makes this a rare example of sympatric speciation in the fossil record. The ability of Z. modesta to live as an epibiont on other filter feeders may have contributed to the longevity of species. This niche partitioning likely played a key role in the Ordovician Radiation, although the role of brachiopods as epibiota remains understudied and the fossil record of these direct associations remains poor.
Further research on the internal morphology of the early spire-bearing brachiopods may reveal how the evolution of their distinctive spiralia may have initially occurred. This seems to have been a key evolutionary innovation because the atrypides and spiriferides dominated shallow marine seas of the later Silurian and Devonian, but it remains unclear how and why the characteristic calcified lophophore supports of these lineages evolved.
Acknowledgments
B. Hussaini and M. Hopkins at the AMNH, C. Schwalbach and B. Hunda at CMC, J. Jin at Western University, and T. Adrain at the University of Iowa all provided loans of brachiopods for this study. J.-B. Caron and M. Akrami at the Royal Ontario Museum provided photos of the type of Z. richmondensis for comparison with other species. R. Fine of the Dry Dredgers in Cincinnati provided valuable information on the distribution of various species of Zygospira in the Cincinnati region. We thank two anonymous reviewers and editors S. Zamora and Z. Zhang for their comments on the initial submission of this manuscript. This work was funded by an NSERC Discovery Grant to Sproat and a Mitacs Indigenous Student Research Award to McLeod. This paper is a contribution to IGCP 735: Rocks and the Rise of Ordovician Life.


























