Introduction
Cnidarians have a geological record extending back to the Ediacaran (Van Iten et al., Reference Van Iten, Marques, Leme, Pacheco and Simões2014, Reference Van Iten, Leme, Pacheco, Simões, Fairchild, Rodrigues, Galante, Boggiani and Marques2016a; Liu et al., Reference Liu, Matthews, Menon, McIlroy and Brasier2014; Grazhdankin, Reference Grazhdankian2016; Han et al., Reference Han, Zhang and Komiya2016; Dzik et al., Reference Dzik, Baliński and Sun2017), but the heavily calcified groups of tabulate and rugosan corals that dominate the Paleozoic fossil record first appear in the Ordovician (Scrutton, Reference Scrutton1997, Reference Scrutton1999). There are, however, many mineralized Cambrian fossils, particularly in the early Cambrian, that have been considered as possible anthozoans, but their relationships to the major zoantharian and octocorallian groups are uncertain (Zhuravlev et al., Reference Zhuravlev, Debrenne and Lafuste1993; Scrutton, Reference Scrutton1997, Reference Scrutton1999; Debrenne and Reitner, Reference Debrenne and Reitner2001; Han et al., Reference Han, Zhang and Komiya2016). Many of these mineralized taxa were informally grouped together as Coralomorpha by Jell (Reference Jell1984; corallomorphs of Zhuravlev et al., Reference Zhuravlev, Debrenne and Lafuste1993). In reviewing the Cambrian record, Scrutton (Reference Scrutton1997, Reference Scrutton1999) concluded that most coralomorphs represented independent, short-lived calcification events in various lineages of anemones and were unrelated to the later tabulate and rugose corals. Scrutton (Reference Scrutton1997, p. 199) proposed the Order Tabulaconida (inadvertently given as Tabuliconida by Peel, Reference Peel2011) for mainly early Cambrian undisputed corals, such as Tabulaconus Handfield, Reference Handfield1969; Flindersipora Lafuste in Lafuste et al., Reference Lafuste, Debrenne, Gandin and Gravestock1991; Moorowipora Fuller and Jenkins, Reference Fuller and Jenkins1994; Arrowipora Fuller and Jenkins, Reference Fuller and Jenkins1995; Yaworipora Zhuravlev, Reference Zhuravlev1999; and Blinmanipora Fuller and Jenkins, Reference Fuller and Jenkins2007. Hicks (Reference Hicks2006) listed 16 taxa of coralomorphs while describing Harklessia Hicks, Reference Hicks2006 from Nevada.
Most early Cambrian coralomorphs are colonial, but Jell and Jell (Reference Jell and Jell1976) described abundant specimens of a cup-shaped coral from the Coonigan Formation, First Discovery Limestone Member (Cambrian Series 3, Stage 5), of New South Wales, Australia, as Cothonion sympomatum Jell and Jell, Reference Jell and Jell1976. This operculate, septate, coral reproduced by budding of daughter corallites from the parent corallum and was placed in a new zoantharian Order Cothoniida by Oliver and Coates (Reference Oliver and Coates1987; see also Scrutton, Reference Scrutton1997). Peel (Reference Peel2011) described Cothonion sympomatum from the Paralleldal Formation (Cambrian Series 2, Stage 4) of southern Peary Land, North Greenland. Coralla of Tretocylichne perplexa Engelbretsen, Reference Engelbretsen1993 from the Murrawong Creek Formation (Cambrian Series 3, Drumian) of New South Wales often display an octagonal form and weak septation, while the holdfast is often perforated into the calice (Engelbretsen, Reference Engelbretsen1993).
Cambroctoconus orientalis Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011 from the Changhia Formation (Cambrian Series 3, Drumian) of Shandong Province, China developed a cup-shaped corallum reminiscent of later zoantharian corals. Its octagonal calice and the eight-fold symmetry of septa promoted comparison with octocorallians and staurozoans, but Cambroctoconus was interpreted by Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, Reference Park, Kihm, Woo, Zhen, Engelbretsen, Hong, Choh and Lee2016) as a stem-group cnidarian (Fig. 1.1, 1.5). Geyer et al. (Reference Geyer, Peel, Streng, Voigt, Fischer and Preuße2014) described a second species, Cambroctoconus kyrgyzstanicus Peel in Geyer et al., Reference Geyer, Peel, Streng, Voigt, Fischer and Preuße2014, from Kyrgyzstan (Cambrian Series 3, Stage 5; Fig. 1.2, 1.4, 1.6), and drew comparisons with Tretocylichne perplexa from Australia. Park et al. (Reference Park, Kihm, Woo, Zhen, Engelbretsen, Hong, Choh and Lee2016) described a third species, Cambroctoconus coreanensis Park et al., Reference Park, Kihm, Woo, Zhen, Engelbretsen, Hong, Choh and Lee2016, from the Daegi Formation (Cambrian Series 3, Drumian) of Korea and discussed the relationship of this proposed group of stem-group cnidarians with crown-groups.
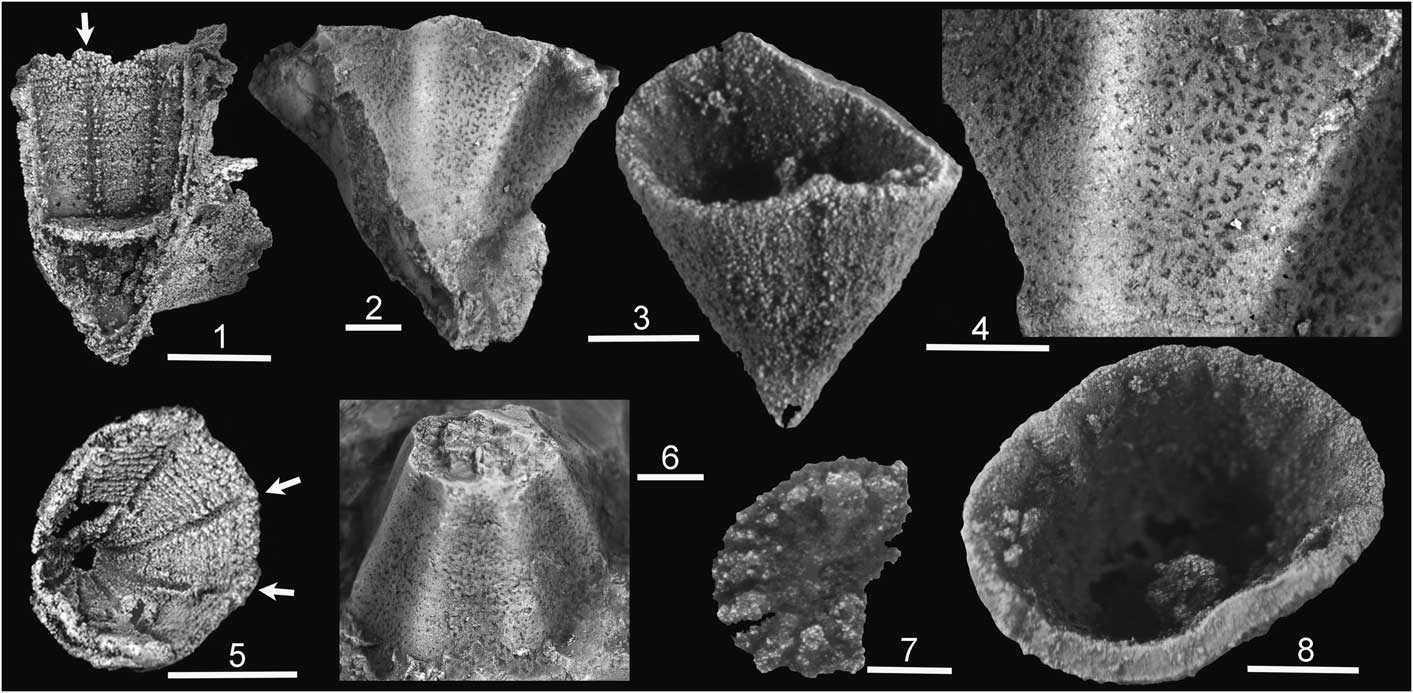
Figure 1 Cambrian cnidarians. (1, 5) Cambroctoconus orientalis Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011 from the Changhia Formation (Cambrian Series 3, Drumian) of Shandong Province, China, with arrows indicating channels between paired septa on the silicified calice wall, SNUP 7011 of Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011), now KOPRIF 17011 (1), SNUP 7013 of Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011), now KOPRIF 17013 (5); photos: Tae-Yoon Park. (2, 4, 6) Cambroctoconus kyrgyzstanicus Peel in Geyer et al., Reference Geyer, Peel, Streng, Voigt, Fischer and Preuße2014, from Kyrgyzstan (Cambrian Series 3, Stage 5) showing porous corallum wall, FG 596/XII/034a, holotype (2, 4), FG 596/XII/007d (6). (3, 7, 8) Cothonion sympomatum Jell and Jell, Reference Jell and Jell1976; Peel (Reference Peel2011) from the Paralleldal Formation (Cambrian Series 2, Stage 4) of North Greenland. MGUH 29268 from GGU sample 271907, septate inner surface of operculum (7), MGUH 29263 from GGU sample 271907, silicified corallum (3, 8). Scale bars: 1 mm, except (1, 5)=5 mm.
Cambroctonus koori new species, described herein from the Henson Gletscher Formation (Cambrian Series 2–3) of North Greenland (Fig. 2), represents the first record of Cambroctoconus from the Laurentian paleocontinent. In contrast to previously described species of Cambroctonus, the new material from Greenland is mainly preserved as phosphatized internal molds etched free from limestone samples by the use of weak acids, whereas specimens of other species are preserved in calcium carbonate (C. kyrgyzstanicus and C. orientalis) or as silica replicas (C. orientalis).
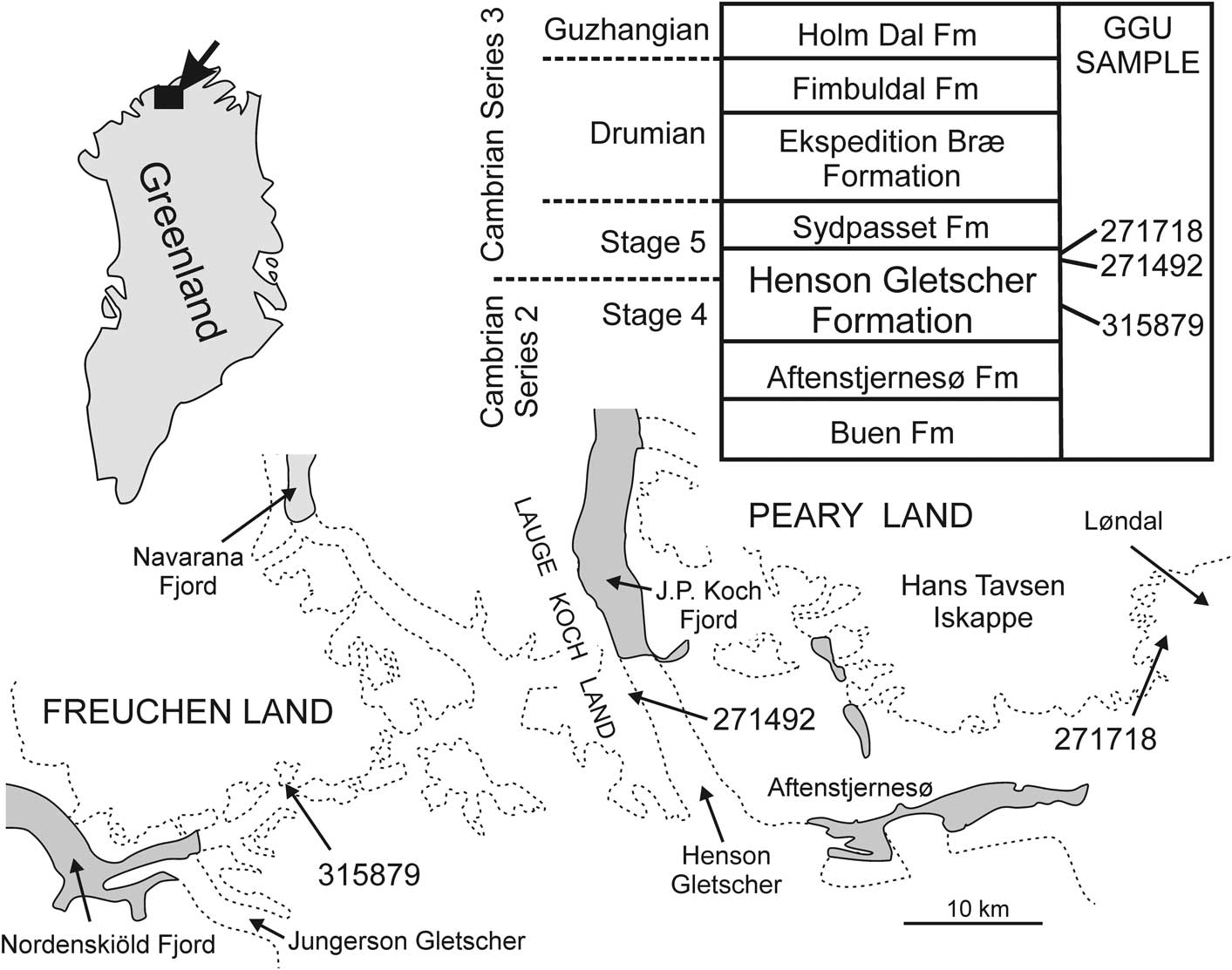
Figure 2 Derivation of samples yielding Cambroctoconus koori n. sp.
Together, the three preservational modes provide a relatively complete picture of the skeletal morphology of Cambroctoconus. Specimens preserved in calcium carbonate permit examination of the corallum wall and calical morphology, especially the thin, paired septa (Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, fig. 2), using the classical thin-section techniques employed by coral researchers. The corallum is also well preserved in silicified specimens of C. orientalis (Fig. 1.1, 1.5). While finer details of septa are not retained in these, budding patterns and the overall form and rejuvenation of the coral occurrences are evident (Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, fig. 1). The phosphatization of C. koori described herein provides an added dimension in that the porous structure characteristic of the corallum wall and septa is made clearly visible by the etching process. Unfortunately, variation in the degree of phosphatization between individual specimens also hinders interpretation. The entire corallum may be replaced such that its outer surface is preserved (Fig. 3.10), or the interior surface of the calice may be molded with varying degrees of penetration of the pore system (compare Fig. 3.6 and Fig. 4.6). In exceptional cases, the pore system is reproduced with great fidelity (Fig. 4.1–4.4).

Figure 3 Cambroctoconus koori n. sp., Henson Gletscher Formation, North Greenland; Cambrian Series 3, Stage 5, Ptychagnostus gibbus Biozone. All specimens from GGU sample 271718 unless stated. (1) PMU 31703, internal mold with septal grooves arrowed; (2) PMU 31704, internal mold, rejuvenated specimen; (3, 4) PMU 31705, internal mold, holotype; (5) PMU 31706, internal mold from GGU sample 271492 with septal groove arrowed; (6, 13) PMU 31707, internal mold in lateral view (6) with earliest growth stage lacking septal grooves, (13) plan view of calice with phosphatic coatings of now-dissolved septa; (7, 8) PMU 31708, internal mold of flattened basal surface within calice with paired septal grooves arrowed; (9, 11) PMU 31709, internal mold of calice in basal (9) and oblique (11) views with eight septa preserved as radial notches; (10) PMU 31710, corallum (inverted) with earliest octagonal stage followed by cylindrical stage; (12) PMU 31711, internal mold of juvenile attached to shell fragment. Scale bars: 200 µm.

Figure 4 Cambroctoconus koori n. sp., Henson Gletscher Formation, North Greenland; Cambrian Series 3, Stage 5, Ptychagnostus gibbus Biozone unless stated. All specimens from GGU sample 271718 unless stated. (1–4) PMU 31712, internal mold viewed from base (broken away from substrate) showing eight principal septa preserved as canals and infilled pore canals; arrows a and b locate equivalent points in (1, 4) and (2, 3), respectively; (5, 6) PMU 31718, internal mold from GGU sample 315879 (Cambrian Series 2, Stage 4) showing eight-fold arrangement of primary septa as ridges on basal surface (5) and continuous ridges (arrowed) separated by areas with infilled pores on the lateral surfaces (6); (7) PMU 31713, lateral view of internal mold showing septal ridges and infilled pores; (8) PMU 31714, lateral view of internal mold showing septal grooves and infilled pores; (9) PMU 31715, lateral view of internal mold; (10) PMU 31716, oblique calical view showing radial channels on floor of calice; (11) PMU 31717, oblique calical view showing broad ridges and narrow channels on base of calice; arrow indicates introduction of new channel. Scale bars: 200 µm, except (3, 4)=100 µm.
Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011) commented that the pore system in Cambroctoconus was a non-cnidarian feature, although mural and septal pores are described in zoantharians and octocorallians (Bayer, Reference Bayer1956; Hill, Reference Hill1981). While its octahedral symmetry encouraged morphological comparison with octocorals and staurozoans, Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011) placed Cambroctoconus at a lower phylogenetic level, as a stem-group cnidarian, following cladistic analysis. The present paper compares the pore system of Cambroctoconus with minute tubules in the coenenchyme of octocorals, the gastrodermal solenia, leading to the suggestion that Cambroctoconus may represent an isolated calcification event in the early history of Octocorallia.
Geological background and material
All described specimens are derived from samples collected from the Henson Gletscher Formation (early–middle Cambrian; Cambrian Series 2, Stage 4–Series 3, Stage 5) in North Greenland (Fig. 2) in the course of regional mapping campaigns (1978–80 and 1984–85) organized by the Geological Survey of Greenland (Peel and Søderholm, Reference Peel and Sønderholm1991). The formation is dominated by thinly bedded, sooty black, limestones, dolostones, and shales with concretions, and lenses and strings of black chert; it was deposited in an off-platform setting during a period of relative lowstand of sea level (Higgins et al., Reference Higgins, Ineson, Peel, Surlyk and Sønderholm1991; Ineson and Peel, Reference Ineson and Peel1997; Geyer and Peel, Reference Geyer and Peel2011). A tripartite sub-division of the formation is apparent in most sections, with lower and upper dark recessive units separated by a prominent median unit of pale sandstones. Blaker and Peel (Reference Blaker and Peel1997) and Geyer and Peel (Reference Geyer and Peel2011) described rich trilobite faunas (Cambrian Series 2, Stage 4) from the Henson Gletscher Formation, while Babcock (Reference Babcock1994) and Robison (Reference Robison1984) described trilobites of Cambrian Series 3 age. Peel et al. (Reference Peel, Streng, Geyer, Kouchinsky and Skovsted2016) described a diverse shelly fauna from strata with Ovatoryctocara granulata (Cambrian Series 2–3 boundary interval) in Løndal (Fig. 1).
GGU sample 315879 was collected by F. Rolle in July 1984 from the lower Henson Gletscher Formation, occurring below the prominent white sandstone unit (Ineson and Peel, Reference Ineson and Peel1997; Geyer and Peel, Reference Geyer and Peel2011) on a nunatak in southern Freuchen Land (82°10.5'N, 42°09'W; Fig. 2); this is locality 3 of Peel (Reference Peel2015, fig. 1). Trilobite faunas described by Blaker and Peel (Reference Blaker and Peel1997) from southern Freuchen Land indicate Cambrian Series 2, Stage 4.
GGU samples 271492 and 271718 were collected from above the median pale sandstone unit in dark limestones, dolostones, and shales of the upper Henson Gletscher Formation, assigned to the Ptychagnostus gibbus Biozone (Robison, Reference Robison1984; Babcock, Reference Babcock1994; Ineson and Peel, Reference Ineson and Peel1997; Clausen and Peel, Reference Clausen and Peel2012; Cambrian Series 3, Stage 5). GGU sample 271492 was collected by J.S. Peel on June 25, 1978 in southern Lauge Koch Land (82°10'N, 40°24'W; Fig. 2) from 5 m below the top of the formation (total thickness 65 m) in its type section, immediately above a prominent mass-flow bed (Ineson and Peel, Reference Ineson and Peel1997, fig. 31). GGU sample 271718 was collected by J.S. Peel on July 15, 1978 in Løndal, Peary Land (82°17.5'N, 37°03'W; Clausen and Peel, Reference Clausen and Peel2012, fig. 1; Fig. 2) from the uppermost 1 m of the formation.
Repositories and institutional abbreviations
GGU prefix indicates samples collected under the auspices of Grønlands Geologiske Undersøgelse (Geological Survey of Greenland, now the Geological Survey of Denmark and Greenland, Copenhagen). Illustrated specimens are deposited in the geological collections of the Technische Universität Bergakademie Freiberg, Germany (FG prefix), the geological type collection of the Natural History Museum of Denmark (MGUH prefix), and the paleontological type collection of the Museum of Evolution, Uppsala University, Sweden (PMU prefix). Specimens stated by Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011) to be deposited in the Seoul National University Museum paleontological collections, Korea (SNUP prefix) have been transferred to the Korea Polar Research Institute (KOPRIF prefix).
Systematic paleontology
Phylum Cnidaria Verrill, Reference Verrill1865
Subphylum Anthozoa Ehrenberg, Reference Ehrenberg1834
? Class Octocorallia Haeckel, Reference Haeckel1866
Order Cambroctoconida new order
Diagnosis
Cup-shaped to cylindrical calcareous skeleton, which is usually octagonal in cross-section and often displays a holdfast. Uniformly spaced septa commonly extend inwards from the inner wall in an eight-fold pattern; when present, they vary in expression from rudimentary to consisting of a pair of closely juxtaposed thin blades. Skeleton and septa perforated by a fine meshwork of irregular vermiform pores.
Remarks
The diagnostic characters were noted by Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, Reference Park, Kihm, Woo, Zhen, Engelbretsen, Hong, Choh and Lee2016) in the description of Cambroctoconus. No explanation was offered for the pore structure of the skeleton and septa, which Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, p. 4) considered to be a non-cnidarian character. Park et al. (Reference Park, Kihm, Woo, Zhen, Engelbretsen, Hong, Choh and Lee2016, p. 829) grouped Australian material of Lipopora Jell and Jell, Reference Jell and Jell1976, Tretocylichne Engelbretsen, Reference Engelbretsen1993, and Cambroctoconus into a ‘stem-group Cnidaria’ on account of the supposed absence of the mesoglea that is characteristic of crown group cnidarians (Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, fig. 4), but this interpretation is rejected. The pore structure of Cambroctoconus is interpreted herein as a reflection of gastrodermal solenia, a characteristic feature of octocorallians, in consequence of which, Cambroctoconida is tentatively placed within the anthozoan Class Octocorallia. While the presence of pores is not established in Tretocylichne on account of its relatively coarse preservation (Engelbretsen, Reference Engelbretsen1993), the octagonal form and eight-fold septation support its assignment to Cambroctoconida. Material of Lipopora is coarsely silicified, but the cylindrical coralla display eight or sixteen septa, which seem to confirm their affinity with Cambroctoconus.
Cambroctoconida (Cambrian Series 2–3) is readily distinguished from other octocorals by the massively calcified, cup-shaped corallum. Soft tissues in present day octocorals are supported by spicules, although a few taxa (e.g., Tubipora Linnaeus, Reference Linnaeus1758; Heliopora de Blainville, Reference Blainville1830; Epiphaxum Lonsdale, Reference Lonsdale1850; and Nanipora Miyazaki and Reimer, Reference Miyazaki and Reimer2015) develop calcified tubes or spicular frameworks containing polyps that rise above sometimes massively calcified stolon platforms. Heliopora, Epiphaxum, and Nanipora are grouped together within a separate order Helioporacea Bock, Reference Bock1938, known to occur already in the Cretaceous (Lozouet and Molodtsova, Reference Lozouet and Molodtsova2008), while Tubipora is placed within the alcyonacean octocorals (Pérez et al., Reference Pérez, Neves, Cordeiro, Williams and Cairns2016).
Genus Cambroctoconus Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011
Type species
Cambroctoconus orientalis Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, from the Changhia Formation (Cambrian Series 3, Drumian) of Shandong Province, China.
Remarks
A detailed comparison between Cambroctoconus and the related genera Tretocyclichne and Lipopora was given by Park et al. (Reference Park, Kihm, Woo, Zhen, Engelbretsen, Hong, Choh and Lee2016). In terms of its turbinate corallum and budding pattern of daughter corallites, Cothonion resembles Cambroctoconus, but it lacks the octagonal shape and eight-fold symmetry of septa. Cothonion is also distinguished by its prominent operculum with septa on the inner surface. Apart from the type locality in Australia, Cothonion is only described from the Paralleldal Formation (Cambrian Series 4, Stage 4) of Peary Land, North Greenland (Peel, Reference Peel2011), which is the same age as GGU sample 315879 from southern Freuchen Land (Fig. 1).
Dzik (Reference Dzik1993) considered Cothonion to be a rugose coral, but this was rejected by Fedorowski (Reference Fedorowski1997). As is the case with cambroctoconids, Cothonion was considered to represent one of several independent calcification events in anthozoan evolutionary history by Scrutton (Reference Scrutton1997, Reference Scrutton1999; see also Oliver and Coates, Reference Oliver and Coates1987 and Peel and McDermott, Reference Peel and McDermott2016).
Aploconus Debrenne et al., Reference Debrenne, Gandin and Gangloff1990 from Cambrian Series 2, Stage 4, of Nevada differs in that the wall of its simple conical skeleton is laminated (Zhuravlev et al., Reference Zhuravlev, Debrenne and Lafuste1993).
Cambroctoconus koori new species
Holotype
PMU 31705 from GGU sample 271718, upper Henson Gletscher Formation, Ptychagnostus gibbus Biozone, Cambrian Series 3, Stage 5, Løndal, Peary Land (Fig. 2).
Paratypes
PMU 31703, 31704, 31707–31717 from GGU sample 271718, Løndal, Peary Land; PMU 31706 from GGU sample 271492, Lauge Koch Land; upper Henson Gletscher Formation, Ptychagnostus gibbus Biozone, Cambrian Series 3, Stage 5. PMU 31718 from GGU sample 315879, Freuchen Land, Henson Gletscher Formation, Cambrian Series 3, Stage 4.
Other material
More than 30 specimens from GGU sample 271718.
Diagnosis
A small species of Cambroctoconus in which the corallum is variable in shape, but most commonly turbinate, becoming cylindrical in later growth stages; octohedral form only rarely developed, budding not observed.
Occurrence
Currently known only from the Henson Gletscher Formation of North Greenland.
Description
The description is based on phosphatized material, both in the form of phosphatized coralla and molds of the calice, showing varying degrees of phosphatization. The corallum is highly variable in shape, ranging from turbinate (Fig. 3.3, 3.4) and trochoid (Fig. 4.9), both of which may become cylindrical in later growth stages (Figs. 3.10, 4.6), to patellate (Fig. 4.1, 4.2). Maximum preserved height is 3 mm, in cylindrical forms. One specimen is attached to a shell fragment (Fig. 3.12), but the flattened or broken base of several coralla (Fig. 4.8) suggests that this was often the case.
The holotype is turbinate, with a shallowly convex lateral profile (Fig. 3.3, 3.4); calical margins of the corallum are not preserved. The base is pointed, without an obvious attachment scar, although such a scar might not be recognized on an internal mold. Eight rounded ridges (width 100–150 µm) are equally disposed over the specimen surface, rising abruptly from the floor of intervening depressions, which increase in width (circumferential) away from the base, as the corallum expands. The depressions are filled with vermiform pore-infillings (diameter 20–50 µm) that arise both from the floor of the depressions and the eight ridges. The pore-fillings are well ordered, labyrinthine in form, and may branch. Near the base, pore-fillings also extend out abaxially from the corallum beyond the outer surface of the rounded ridges.
The turbinate holotype clearly represents an internal mold of the basal portion of the corallum after dissolution of the corallum wall. Thus, the eight rounded ridges represent channels on the inside of the corallum wall comparable to those preserved in silicified coralla of Cambroctoconus orientalis (arrows in Fig. 1.1, 1.5). Pores extended from these channels and from the inner surface of the calice into the corallum wall. The channels in C. orientalis lie between a pair of thin perforated septa (Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, fig. 2), which are not preserved in the holotype of Cambroctoconus koori n. sp. The abaxial extension of pore-infillings from the upper surface of the rounded ridges in the holotype of C. koori n. sp. indicates that the thickness of the corallum wall was at least twice the height of the ridges. The texture of the outer surface of the corallum (Fig. 3.10) suggests that the pores extended to the outer surface of the corallum as in Cambroctoconus kyrgyzstanicus (Fig. 1.2, 1.4).
In other specimens, septa on the outer surface of the phosphatized molds are preserved as grooves in the surface of the mold (Fig. 3.1, 3.5, 3.6). Deep grooves representing septa on the interior of the corallum are impressed into the surface of the mold, with porous areas between. Infilled pores traverse the septa. Septa bifurcate at ~500 µm from the pointed base (Fig. 3.1, 3.5, arrows) and are equated with the thin perforated septa visible in transverse thin-sections of C. orientalis (Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, fig. 2). In silicified specimens of the type species, the raised margins of the eight channels (arrows in Fig. 1.5) indicate the position of these thin perforated septa.
The basal surface of several internal molds is flattened (Figs. 3.7–3.9, 3.11, 4.5), possibly representing a broadening of the area of attachment (Fig. 3.5), the presence of a transverse tabula within the corallum (as seen in the type species, Fig. 1.1), or a basal plug of shell material. The mold surface carries eight radiating rounded ridges, comparable to those seen in the holotype (Fig. 3.3), but secondary ridges are quickly interspersed between the primary eight (Fig. 4.5, 4.6). Thus, 16 grooves (four of which are arrowed in Fig. 3.7) crossed by pore-infillings on the internal mold correspond to 16 pore-traversed septa on the corallum interior.
In one specimen, viewed from the base (Fig. 4.1–4.3), the attachment surface is broken away; it is 1.2 mm in diameter. A circular inner zone (Fig. 4.3), forming half the specimen diameter, is surrounded by an irregular network of infilled pores (Fig. 4.1–4.4) that represent two growth iterations (Fig. 4.2). The inner zone displays eight equidistant tubes, each of which represents a now-dissolved solid structure ~80 µm in diameter, but crossed by infrequent pores. The tubes slope radially outwards in this basal view, with steep inclination, as indicated by the impression of their inner margin on the central area, forming a similar pattern to that seen on the basal termination in Figure 4.5. Fine pores ~10 µm in diameter are concentrated in the central part of the inner zone and along the eight radiating ridges between the eight tubes (Fig. 4.3). The pore arrangement in the outer zone is labyrinthine; pores are interconnected, but they are irregular in shape, orientation and diameter, the latter varying from ~5 µm to 30 µm (Fig. 4.4). There is a tendency for individual pores to swell at junctions with other pores.
Budding from the corallum has not been observed, but several specimens show rejuvenescence of the corallum (Fig. 3.2). Several internal molds lack septal grooves in their earliest growth stage (Fig. 3.6).
Etymology
From koori (Greenlandic), basket.
Remarks
Variation in the morphology of the preserved internal molds of Cambroctoconus koori n. sp. suggests that phosphatization was not a uniform process. While some specimens show a high degree of penetration of the porous wall of the corallum and detailed moldic reproduction (Fig. 4.1–4.4), others show more massive textures suggesting simple or even repeated molding of the calice or a lack of penetration of the pore complex (Fig. 3.5). The differences may also reflect pre- or postmortem closure of pore spaces by carbonate.
With a total preserved height of 3 mm, internal molds of C. koori n. sp. are much smaller than the type species C. orientalis (height 11–13 mm; Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011), C. coreanensis (height 11–16 mm; Park et al., Reference Park, Kihm, Woo, Zhen, Engelbretsen, Hong, Choh and Lee2016), or C. kyrgyzstanicus (height 7 mm; Peel in Geyer et al., Reference Geyer, Peel, Streng, Voigt, Fischer and Preuße2014). However, the extent of phosphatization within coralla of C. koori n. sp. is not well known in most specimens, and it is probable that phosphatization was restricted often just to the deepest portions of the calice. Thus, the original calcareous coralla of many specimens of C. koori n. sp. may have been higher than the preserved internal molds.
Several specimens lack indications of septa on the outer wall of the corallum and this surface, as preserved, may approximate to the true outer wall of the corallum (Figs. 3.10, 4.10, 4.11). Thus, phosphatization has replaced the entire specimen rather than just forming a mold of the interior. In the largest available specimen (Fig. 3.10), the turbinate earliest growth stage is octagonal in cross-section (not a common feature in C. koori n. sp.) and is succeeded, following a clear transverse fracture, by a cylindrical latest growth stage, which is interpreted as the outer surface of the corallum. The floor of the calice is preserved in several of the specimens that lack indications of septa on the outer corallum wall (Fig. 4.10, 4.11). Radiating and bifurcating ridges (Fig. 4.11, point of bifurcation arrowed) can be equated with the bifurcating septal grooves in other specimens (Fig. 3.1, arrows). However, deep septal grooves in the outer surface (Fig. 3.6) correspond to ridges on the calice interior (Fig. 3.13), clearly demonstrating that the entire specimen is an internal mold of the calice.
Apart from greater size, C. orientalis and C. kyrgyzstanicus differ from C. koori n. sp. in the much more strongly expressed octagonal cross-section of their coralla. An octagonal form has been observed only rarely in specimens from Greenland (Fig. 3.10) where the majority have a circular cross-section. To some extent, this may reflect a difference in calcification between the outer wall of the corallum and the calice, but thin-sections illustrated by Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, fig. 2) indicate that the octagonal form of C. orientalis is maintained within the calice. Apart from the difference in cross-section, both C. orientalis and C. koori n. sp. show a similar range in the overall shape of the corallum.
Specimens of C. orientalis illustrated by Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, fig.1) show budding from the calice brim and from the corallum walls, with stacks of successively formed corallites. Several daughter corallites may arise from a single parent, but connection between polyps through the walls is not seen. Budding from the corallum walls is not known in C. koori n. sp., but rejuvenation is seen (Fig. 3.2), as illustrated also by Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, fig.1i, 1k, 1l) in the type species. Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, supplementary figs. S4, S5) illustrated large numbers of closely juxtaposed specimens of C. orientalis on a bedding plane, which were interpreted as forming a colony. It is not known if these specimens represent just a physical association of individuals, either in life or death, or formed a colony of biologically connected individuals. Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011) referred to the associations as colonies, but their comment that the lack of shared colonial tissue (coenenchyme) was a point of difference with Anthozoa suggests just association rather than connection of individuals.
Cambroctoconus coreanensis, from the Daegi Formation (Cambrian Series 3, Drumian) of Korea (Park et al., Reference Park, Kihm, Woo, Zhen, Engelbretsen, Hong, Choh and Lee2016), differs from C. koori n. sp. in its slender, slightly sinuous coralla, but its internal structures are not well known.
Eight-fold symmetry of Cambroctoconus
The eight-fold symmetry in Cambroctoconus orientalis, expressed by the octagonal corallum and eight pairs of septa, is a characteristic feature of octocorals and staurozoans, but it is not confined to cnidarians. It is also characteristic of ctenophores, which were already well represented in the Cambrian (Conway Morris and Collins, Reference Conway Morris and Collins1996; Hou et al., Reference Hou, Aldridge, Bergström, Siveter, Siveter and Feng2004; Ou et al., Reference Ou, Xiao, Han, Sun, Zhang, Zhang and Shu2015). Apart from the eight comb rows, the symmetry of ctenophores is clearly demonstrated by the plated apical organ of a sclerotized species from the Chengjiang Lagerstätten (Ou et al., Reference Ou, Xiao, Han, Sun, Zhang, Zhang and Shu2015, figs. 1K, 1L). Eight spiraling structures characterize the problematic Eoandromeda Tang et al., Reference Tang, Yin, Bengtson, Liu, Wang and Gao2008 from the Ediacaran of China and Australia (Tang et al., Reference Tang, Yin, Bengtson, Liu, Wang and Gao2008; Zhu et al., Reference Zhu, Gehling, Xiao, Zhao and Droser2008). Haootia quadriformis Liu et al., Reference Liu, Matthews, Menon, McIlroy and Brasier2014 from the Ediacaran of Newfoundland, an impression of a soft-bodied organism with a holdfast, shows four bifurcating branches and was compared to staurozoans and interpreted as a stem group medusozoan (Liu et al., Reference Liu, Matthews, Menon, McIlroy and Brasier2014; Miranda et al., Reference Miranda, Collins and Marques2014).
In detail, the eight-fold symmetry of octocorals has a bilateral component imposed upon it by the development of a ciliated organ (siphonoglyph) at the entrance to the pharynx. Almost all living octocorals are colonial with coenenchymal tissue embracing polyps and stolons that form ribbons or sheets, although a solitary deep-sea taxon was described by Bayer and Muzik (Reference Bayer and Muzik1976). Polyp walls in the dominantly soft body are supported by a collar of calcite spicules in adjacent areas of the coenenchyme and in the axial area of branches. However, domed colonies of Tubipora musica Linnaeus, Reference Linnaeus1758 are supported by a compact skeleton composed of porous radial tubes (1.3–1.8 mm in diameter) and concentric stolonal platforms built of tightly conjoined spicules (Spiro, Reference Spiro1971). In contrast, the blue octocoral Heliopora, and the related Epiphaxum and Nanipora have rigid internal aragonitic skeletons as well as calcite spicules (Bayer, Reference Bayer1992; Miyazaki and Reimer, Reference Miyazaki and Reimer2015). Octocorals have a poor geological record, but reports of supposed gorgonians exist from the Ordovician (Lindström, Reference Lindström1978; Cope, Reference Cope2005). The problematic middle Cambrian Echmatocrinus Sprinkle, Reference Sprinkle1973 has been interpreted both as a crinoid and as an octocoral (Sprinkle, Reference Sprinkle1973; Conway Morris, Reference Conway Morris1993; Sprinkle and Collins, Reference Sprinkle and Collins1998; Ausich and Babcock, Reference Ausich and Babcock1998, Reference Ausich and Babcock2000), but it is quite unlike Cambroctoconus. Isolated octocoral spicules have been recognized from the Silurian (Bengtson, Reference Bengtson1981) and possibly from the Cambrian (Microcoryne Bengtson in Bengtson et al., Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990).
In Epiphaxum septifer Bayer, Reference Bayer1992 a cylindrical calice ~500 µm in diameter illustrated by Bayer (Reference Bayer1992, fig. 30) has eight robust radial septa that alternate with eight internal rows of large pores through which solenia pass; the mesenteries are believed to be attached to the septa. In shape and size, the septa are similar to the septal notches in one illustrated internal mold of C. koori n. sp. (Fig. 3.9, 3.11), although this represents only one preservational variety in the Greenland material. Similar septa are also present in Heliopora coerulea, but may vary in number from eight in the deepest part of the calice up to 16 at higher levels (Bayer, Reference Bayer1992). Epiphaxum and Heliopora do not develop the delicate paired septa visible in C. orientalis (Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, fig. 2) and inferred in C. koori n. sp. (Fig. 3.7, 3.8, arrows).
Among other cnidarians, Dzik et al. (Reference Dzik, Baliński and Sun2017) noted biradially disposed sets of eight septa-like structures in non-calcified Ordovician specimens of Sphenothallus Hall, Reference Hall1847, a genus known from the lower Cambrian (Zhu et al., Reference Zhu, Van Iten, Cox, Zhao and Erdtmann2000; Peng et al., Reference Peng, Babcock, Zhao and Yang2005; Muscente and Xiao, Reference Muscente and Xiao2015) and widely reported in younger Paleozoic strata (Van Iten et al., Reference Van Iten, Cox and Mapes1992, Reference Van Iten, Zhu and Collins2002, Reference Van Iten, Marques, Leme, Pacheco and Simões2014, Reference Van Iten, Leme, Pacheco, Simões, Fairchild, Rodrigues, Galante, Boggiani and Marques2016a, Reference Van Iten, Muir, Simões, Leme, Merques and Yoder2016b). Dzik et al. (Reference Dzik, Baliński and Sun2017) noted the similarity of Sphenothallus to the polyps of present day coronate scyphozoans (Medusozoa).
Pore structure of Cambroctoconus
The pore structure characteristic of Cambroctoconus is highly unusual within calcified Cnidaria, although pores occur in the septa of Ordovician–Devonian calostyline rugose corals (Lindström, Reference Lindström1870; Kaljo and Reiman, Reference Kaljo and Reiman1958; Weyer, Reference Weyer1973; Hill, Reference Hill1981; Elias, Reference Elias1986), the septa and walls of tabulate corals (Hill, Reference Hill1981), in some scleractinian corals (Stolarski, Reference Stolarski2000; Stolarski and Roniewicz, Reference Stolarski and Roniewicz2001), and in rare calcified octocorals such as Epiphaxum and Nanipora (Bayer, Reference Bayer1992; Miyazaki and Reimer, Reference Miyazaki and Reimer2015). In the widespread Silurian–Devonian rugosan genus Calostylus Lindström, Reference Lindström1868, the combination of pores, synapticulae, and irregular, retiform septa may produce a peripheral porous structure that partially replaces the regular pattern of radial septa, and is reminiscent of the pore structure in Cambrocotoconus. In Helminthidium Lindström, Reference Lindström1882 from the Silurian of Gotland, this porous zone occupies the entire area within a prominent epitheca, but the pores may be visible externally in Calostylis due to the frequent distal lack of an epitheca (Kaljo and Reiman, Reference Kaljo and Reiman1958). In this respect, Calostylis resembles Cambroctoconus (Fig. 1.4), but the abundant septa and domed tabulae of the former find no equivalence in Cambroctoconus.
In its most clearly expressed form (Fig. 4.1–4.4), there is superficial resemblance of the pore structure of C. koori n. sp. to the stereom of echinoderms, but the latter is much more regular, better ordered, and with greater connectivity of the stromal tissue, as demonstrated by Clausen and Peel (Reference Clausen and Peel2012) in their study of well-preserved echinoderm plates occurring together with C. koori in GGU sample 271718. Smith and Jell (Reference Smith and Jell1990) described a plate with poorly ordered stereom from the Beetle Creek Formation (Cambrian Series 3) of Queensland, but it has no morphological resemblance to coralla of Cambroctoconus. Echinoderm stromal tissue consists of muscles and ligaments connecting adjacent skeletal elements, a function that is readily discounted in Cambroctoconus.
Archaeocyathan sponges are abundant in the early Cambrian and combine radial septa and pores in their skeletons; they are usually studied as thin sections of their calcareous cups. A small assemblage of archaeocyaths is known from the Paralleldal Formation (Cambrian Series 2, Stage 4) in Peary Land, in strata equivalent to those yielding the problematic zoantharian Cothonion (Debrenne and Peel, Reference Debrenne and Peel1986; Peel, Reference Peel2011), but there are no co-occurrences with Cambroctoconus koori n. sp. Septa in the intervallum of Archaeocyathus pearylandensis Debrenne and Peel, Reference Debrenne and Peel1986 are porous and undulating, especially abaxially, but the septa are many times more numerous than in Cambroctoconus and there is no suggestion of an octagonal form (Debrenne and Peel, Reference Debrenne and Peel1986, fig. 8a).
Internal molds of archaeocyaths are rarely illustrated, but a juvenile specimen figured by Wrona (Reference Wrona2004, fig. 25I) shows a superficial resemblance to some specimens of C. koori n. sp. (Fig. 3.6), with narrow septa and coarse tubercles reflecting pores through the outer wall. Coincidently, eight septa are present in this specimen at this size (diameter 3.5 mm), but these culminate adaxially at an inner wall, which delimits an octagonal central cavity; much larger numbers of septa are present in larger specimens and in other taxa. The pores in Wrona’s (Reference Wrona2004) specimen are much larger and simpler in form than the irregular vermiform pore-infillings seen in C. koori n. sp. (Fig. 3.3, 3.4). However, vermiform pores may be developed in many ‘irregular’ archaeocyaths, including the rare Cambrian Series 3 (Drumian) representative (Wood et al., Reference Wood, Evans and Zhuravlev1992), and an inner wall may be lacking (Hill, Reference Hill1972; Debrenne et al., Reference Debrenne, Zhuravlev and Kruse2000). While preservational differences hinder comparison between C. koori n. sp. and archaeocyaths in their typical preservation, thin sections of C. orientalis and C. kyrgyzstanicus show a thick outer wall and long septa unlike archaeocyaths (Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011; Geyer et al., Reference Geyer, Peel, Streng, Voigt, Fischer and Preuße2014).
Anthaspidellid sponges, such as Fieldospongia Rigby, Reference Rigby1986 and Rankenella Kruse, Reference Kruse1983, may appear superficially similar to the internal molds of Cambroctoconus koori n. sp., but their framework is composed of interlocking spicules rather than a network of pores traversing septa (Kruse and Zhuravlev, Reference Kruse and Zhuravlev2008; Botting and Peel, Reference Botting and Peel2016; Lee et al., Reference Lee, Woo and Lee2016).
Pores in archaeocyathans and other sponges are open canals for the passage of water currents with food particles into the central cavity, a role that is difficult to reconcile with the calical cavity of a supposed cnidarian containing a tentaculate polyp.
The coenenchyme of octocorals is penetrated by a complex series of tubules (solenia), which connect adjacent polyps and distribute nutrients. The coenenchyme is often heavily supported by calcareous spicules, especially around the polyps (Bayer, Reference Bayer1956), but in Epiphaxum the polyps possess a solidly calcified, tubular outer wall with 16 rounded ridges that alternate with u-shaped channels of similar width. However, only half this number is present in a juvenile specimen of Epiphaxum breve Bayer, Reference Bayer1992 (see Bayer, Reference Bayer1992, fig. 14), giving an octoradial form similar to Cambroctoconus. The channels are occupied by solenia, which pass through solenial pores ~30–40 µm in diameter, but are accompanied by vertical rows of tiny pores (diameter 10 µm). The large pores in the 16 external channels join laterally so that only eight rows of pores are present internally, alternating with the mesenteries. In a calice of Epiphaxum septifer illustrated by Bayer (Reference Bayer1992), the eight internal rows of large pores alternate with radial septa. Solenia occupy galleries 100–200 µm in diameter in the calcified coenenchyme of Nanipora kamurai, where the outer surface of the calcareous polyp tubes is not ridged, as in Epiphaxum, but covered with 5 µm diameter pores, possibly desmocytes anchoring soft tissues to the skeleton (Bayer, Reference Bayer1992).
The large solenial pores in the polyp walls of Epiphaxum are more highly organized than those in Cambroctoconus, which compare more closely in form with the system of solenia within the coenenchyme (Bayer, Reference Bayer1956, fig. 134). However, the perforation of the outer wall of the corallum in Cambroctoconus (Fig. 1.2, 1.4) indicates that the corallum was enveloped by a thin layer of coenenchymal tissue (Fig. 5), as in Nanipora, rather than just an edge zone to the calice, as is the case in solitary rugosan and scleractinian corals. Excellent illustrations of both the aragonite skeleton and the living colony of Nanipora were presented by Miyazaki and Reimer (Reference Miyazaki and Reimer2015).
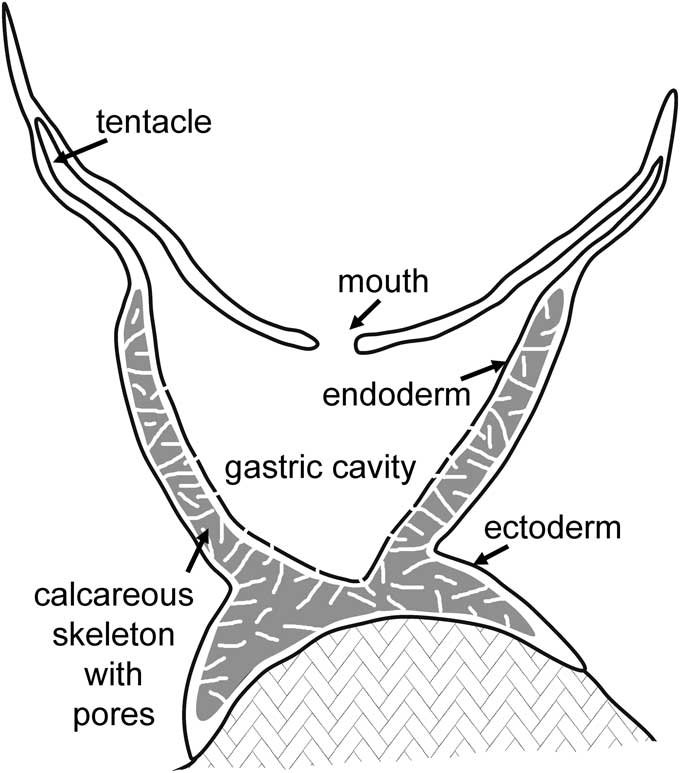
Figure 5 Schematic cross-section of Cambroctoconus koori n. sp. showing development of the porous calcareous skeleton within the mesogleal layer of the coenenchyme.
Systematic position of Cambroctoconus
Similarity of Cambroctoconus with octocorals, suggested by the octagonal cross-section of the corallum and the eight-fold arrangement of septa, is supported by the interpretation of the pores in the walls of the corallum and septa as solenia within the mesoglea of the coenenchyme (Fig. 5). Thus, the corallum is formed as a cup to the polyp by massive calcification around the solenia that in octocorals distribute nutrients between the individual polyps and the coenenchyme. The calcified corallum can be compared in its place and function with the aragonitic skeletons of present day Epiphaxum and Nanipora, which are also perforated by pores, although these are tubular calices within colonial organisms rather than individual conical coralla. The corallum is also analagous with the fused spicular calcite skeletons developed within the mesoglea that serve to elevate the polyps above the stolon in many extant octocorals (calyx of Bayer, Reference Bayer1956). This spicule-based skeleton is particularly prominent in Tubipora. Coralla of Cambroctoconus achieved stability by cementation to the substrate (Fig. 3.12), but the basal surface in Epiphaxum may be formed by a massive carbonate deposit (Bayer, Reference Bayer1992).
There is no preserved record of stolons or other extensions of coenenchymal tissue beyond the coralla in Cambroctoconus, but if present, it is likely that these were not calcified; they may have been spiculate. However, a particularly dense pattern of solenial pores in the basal area of Cambroctoconus (Fig. 4.1–4.4) may suggest enhanced nutrient flow in this area. The solenial pores of Cambroctoconus served to distribute nutrients from the gastric cavity to the mesogleal layer, providing a base for the significant energy investment involved in the production of the calcified skeleton. However, stolons are also lacking in Taiaroa tauhau Bayer and Muzik, Reference Bayer and Muzik1976, the only present day solitary octocoral, individuals of which are secured within the sediment by numerous thread-like holdfasts. The lower part of the cylindrical T. tauhau (acanthostele) is supported by eight prominent ridges reinforced with closely packed sclerites. Despite their extensive spiculation (Bayer, Reference Bayer1956), octocorals have left little trace in the fossil record. Bayer (Reference Bayer1992, figs. 9, 10) illustrated incorporation of calcitic spicules into the aragonite polyp walls of Epiphaxum, but such spicules have not been recognized in Cambroctoconus koori n. sp.
It is possible that individual coralla of Cambroctoconus developed periodically from creeping or sheet-like stolons in similar fashion to the simple, upright, polyps of the present day octocoral Clavularia de Blainville, Reference Blainville1830. However, stolons are not present in Taiaroa tauhau, the only living solitary octocoral (Bayer and Muzik, Reference Bayer and Muzik1976), and evidence of their presence in Cambroctoconus is lacking. Extension of coenenchyme beyond the initial corallum would be a first step to the establishment of the colonial growth characteristic of almost all octocorals. Coralla in Cambroctoconus achieved stability by cementation to the substrate (Fig. 3.12), although the body of T. tauhau is supported by fibrous holdfasts. Budding in Clavularia takes place from the stolons, but daughter zooids in telestacean octocorals are produced directly from the outer coenenchymal wall of the parent, from the anastomosing network of solenia (Bayer, Reference Bayer1973).
Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011) noted that anthozoan polyps arise from the mesoglea. Because budding in Cambroctoconus orientalis took place on both the outside and inside of the parent corallum, they argued that the skeletal wall must have been highly integrated with the soft tissues from which the daughter coralla arose, although similar budding was also described in Cothonion by Jell and Jell (Reference Jell and Jell1976). This observation is consistent with the present interpretation of the corallum wall as formed around the gastrodermal solenia within the mesoglea (Fig. 5), as in the octocoral Epiphaxum. It is supported by the occurrence of secondary calices branching from the primary calice in Epiphaxum, which arise from the mesoglea filling the channels on the outside of the calcified polyp wall (Bayer, Reference Bayer1992). However, Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011) compared the skeletal walls of Cambroctoconus with cnidarians in which the outer corallum wall is a true epitheca deposited from the epidermis and not closely integrated with the mesogleal polyp tissues from which new individuals arose. This comparison was cited as evidence that mesoglea (jelly-like mesenchyme of Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011) was absent in Cambroctoconus, which was considered, therefore, to represent a stage in cnidarian evolution earlier than the acquisition of the mesoglea that is characteristic of crown group cnidarians (Park et al., Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011, fig. 4). Thus, Cambroctoconus was interpreted by Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011) as a stem-group cnidarian. However, the present interpretation that the outer wall in Cambroctoconus formed within the mesoglea (Fig. 5), as is the case with the calcified calice walls in the octocorals Epiphaxum and Nanipora, demonstrates that a mesogleal layer was present in Cambroctoconus, refuting the argumentation proposed by Park et al. (Reference Park, Woo, Lee, Lee, Lee, Han, Chough and Choi2011).
Description of gastrodermal solenia in Cambroctoconus promotes recognition of the three-layer coenenchyme that is diagnostic of cnidarians (endoderm + mesoglea + ectoderm; Fig. 5). In this feature, Cambroctoconus is typically cnidarian and not a pre-mesogleal stem-group representative. When considered together, the octagonal form, eight-fold septal symmetry, and solenial pore system suggest that Cambroctonconus was a basal member of the octocoral clade, and thus represented an early calcification event in the phylogenetic history of Octocorallia.
In traditional morphological and rRNA genomic classification (Collins, Reference Collins2002, Reference Collins2009), Hexacorallia and Octocorallia are sister groups within Anthozoa, which itself is a sister group of Medusozoa. Kayal et al. (Reference Kayal, Roure, Philippe, Collins and Lavrov2013) rejected the monophyly of Anthozoa following phylogenetic analysis based on mitochondrial genomics, and placed Octocorallia closer to Medusozoa. They suggested that Medusozoa (Scyphozoa+Hydrozoa) formed a sister group to Octocorallia, and that these together were a sister group to Hexacorallia (Kayal et al., Reference Kayal, Roure, Philippe, Collins and Lavrov2013). The phylogeny was cited as potentially supporting the ‘polyp before medusa’ hypothesis of cnidarian evolution (Ax, Reference Ax2012), a theme taken up by Dzik et al. (Reference Dzik, Baliński and Sun2017) in the context of the fossil record of the tubular cnidarian Sphenothallus Hall, Reference Hall1847 and related Lower Paleozoic taxa (Tynan, Reference Tynan1983; Van Iten et al., Reference Van Iten, Marques, Leme, Pacheco and Simões2014).
Zapata et al. (Reference Zapata, Goetz, Smith, Howison, Siebert, Church, Sanders, Ames, McFadden, France, Daly, Collins, Haddock, Dunn and Cartwright2015) restated the monophyly of Anthozoa, Octocorallia, and Hexacorallia. They admitted, however, that the combination of the deep history of some crown groups and extensive record of fossil extinction events provides a substantial hinder to the reconstruction of cnidarian phylogeny. It is an obstacle that Cambrian fossil cnidarians, including Cambroctoconus, can help to overcome.
Acknowledgments
H. Agic and A. Kouchinsky made some of the SEM images. J. Botting, A.B. Smith, and A.Yu. Zhuravlev gave valuable suggestions and pointers to literature sources. T.-Y. Park provided illustrations of Cambroctoconus orientalis, information concerning the relocation of museum specimens, and reviewed the submitted manuscript. R. Elias is thanked for information concerning calostyline corals and for reviewing the submitted manuscript.







