Non-technical Summary
Seven species occur in shallow-marine limestone of the Sete Lagoas Formation, Bambuí Group, in Januária, Brazil, including Siphonophycus robustum, Leiosphaeridia crassa, Leiosphaeridia jacutica, Leiosphaeridia minutissima, Leiosphaeridia tenuissima, Germinosphaera bispinosa, and a new species named Ghoshia januarensis. In the lower part of the studied section, these occurrences are common, but only Ghoshia januarensis is found in the upper part. This is likely due to changes in the environment or preservation conditions. The Leiosphaeridia species, especially Leiosphaeridia minutissima, dominates the assemblage of organic-walled microfossils. While most described taxa have long stratigraphic ranges, they are consistent with a terminal Ediacaran age, as indicated by detrital zircon data and tubular fossils (e.g., Cloudina and Corumbella) from the Sete Lagoas Formation.
Introduction
The present work provides an updated taxonomic description and biostratigraphic analysis of organic-walled microfossils from the Sete Lagoas Formation, Bambuí Group, from the countryside of Januária Municipality, Minas Gerais State, Brazil. The studied area is an ancient quarry area with an exposure of nearly 70 m of a continuous succession of mixed carbonate and fine-grained siliciclastic rocks. In addition, the Januária area has been the focus of the chronostratigraphic investigation of the Sete Lagoas Formation since Warren et al. (Reference Warren, Quaglio, Riccomini, Simões, Poiré, Strikis, Anelli and Strikis2014) reported Ediacaran tubular fossils such as Cloudina sp. and Corumbella werneri Hahn et al., Reference Hahn, Hahn, Leonardos, Pflug and Walde1982 from the lower Sete Lagoas Formation in the Januária area.
Several articles on organic-walled microfossils from the Sete Lagoas Formation have been previously published (Sommer, Reference Sommer1971; Simonetti and Fairchild, Reference Simonetti and Fairchild1989, Reference Simonetti and Fairchild2000; Fairchild et al., Reference Fairchild, Schopf, Shen-Miller, Guimarães, Edwards, Lagstein, Li, Pabst and Melo Filho1996, Reference Fairchild, Sanchez, Pacheco and Leme2012; Sanchez and Fairchild, Reference Sanchez and Fairchild2018), describing dozens of species from this unit (Table 1), although Sanchez and Fairchild (Reference Sanchez and Fairchild2018) invalidated one species, Bambuites erichsenii Sommer, Reference Sommer1971. It is important to underscore that the investigation conducted by Fairchild et al. (Reference Fairchild, Schopf, Shen-Miller, Guimarães, Edwards, Lagstein, Li, Pabst and Melo Filho1996) centered on the analysis of silicified carbonaceous microfossils in petrographic thin sections from the Sete Lagoas Formation in the western portion of the São Francisco craton. Specimens illustrated by Fairchild et al. (Reference Fairchild, Schopf, Shen-Miller, Guimarães, Edwards, Lagstein, Li, Pabst and Melo Filho1996) were tentatively compared with seven species, alongside several taxa left under open nomenclature. This study provides a detailed taxonomic description with an analysis of the diversity, abundance, and stratigraphic occurrence of organic-walled microfossils from the Sete Lagoas Formation in the Barreiro section in the Januária area.
Table 1. List of microfossils from the Sete Lagoas Formation, Bambuí Group, published previously and in this study. Articles: 1 = Sommer (Reference Sommer1971); 2 = Marchese (Reference Marchese1974); 3 = Simonetti and Fairchild (Reference Simonetti and Fairchild1989); 4 = Fairchild et al. (Reference Fairchild, Schopf, Shen-Miller, Guimarães, Edwards, Lagstein, Li, Pabst and Melo Filho1996); 5 = Simonetti and Fairchild (Reference Simonetti and Fairchild2000); 6 = Fairchild et al. (Reference Fairchild, Sanchez, Pacheco and Leme2012); 7 = Warren et al. (Reference Warren, Quaglio, Riccomini, Simões, Poiré, Strikis, Anelli and Strikis2014); 8 = Perrella Júnior et al. (Reference Perrella Júnior, Uhlein, Uhlein, Sial, Pedrosa-Soares and Lima2017); 9 = Sanchez and Fairchild (Reference Sanchez and Fairchild2018); 10 = Denezine et al. (Reference Denezine, Adôrno, Do Carmo, Guimarães, Walde, Alvarenga, Germs, Antonietto, Gianfranco and Nunes Junior2022); 11 = this study.

The remarkable diversity of organic-walled microfossils in the Ediacaran Period (Knoll, Reference Knoll1994; Vidal and Moczydłowska-Vidal, Reference Vidal and Moczydłowska-Vidal1997; Huntley et al., Reference Huntley, Xiao and Kowalewski2006) is hypothesized to be associated with the ecological rise of animals (Peterson and Butterfield, Reference Peterson and Butterfield2005), followed by the advent of skeletonized animals as evidenced by fossil cloudinids and corumbellids (Germs, Reference Germs1972; Hahn et al., Reference Hahn, Hahn, Leonardos, Pflug and Walde1982; Hua et al., Reference Hua, Chen, Yuan, Zhang and Xiao2005; Walde et al., Reference Walde, Do Carmo, Guimarães, Vieira, Erdtmann, Sanchez, Adôrno and Tobias2015; Adôrno et al., Reference Adôrno, Do Carmo, Germs, Walde and Denezine2017). The transition from the Ediacaran to the Cambrian Period is marked by a significant turnover of acritarch species (Anderson et al., Reference Anderson, Macdonald, Jones, McMahon and Briggs2017; Grazhdankin et al., Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020; Morais et al., Reference Morais, Fairchild, Freitas, Rudnitzki, Silva, Lahr, Moreira, Abrahão Filho, Leme and Trindade2021). The Cambrian is characterized by a diversification of acanthomorphs species compared with the sphaeromorph dominance in the late Ediacaran (Gaucher and Sprechmann, Reference Gaucher and Sprechmann2009). Acanthomorphic acritarchs define four acritarch assemblage zones to recognize the lower Cambrian on the East European Platform (Moczydłowska, Reference Moczydłowska1991): Asteridium tornatum–Comasphaeridium velvetum Assemblage Zone, Skiagia ornata–Fimbriaglomerella membranacea Assemblage Zone, Heliosphaeridium dissimilare–Skiagia ciliosa Assemblage Zone, and Volkovia dentifera–Liepaina plana Assemblage Zone. These biostratigraphic units have been used to correlate Cambrian successions around the world (Zheng et al., Reference Zheng, Clausen, Feng and Servais2020). Therefore, a systematic study of organic-walled microfossils from the Sete Lagoas Formation not only provides a tool for biostratigraphic correlation but also offers useful data to improve the understanding of Ediacaran evolution.
Geological setting
The Januária Municipality is located in the central part of the São Francisco craton (Fig. 1). The paleogeographic location of the São Francisco craton during the Ediacaran Period has not been precisely constrained, mainly because of poor biostratigraphic and paleomagnetic data. However, Merdith et al. (Reference Merdith, Williams, Collins, Tetley and Mulder2021) placed the São Francisco craton in high latitudes during the Ediacaran Period.

Figure 1. (1) Geological map of the São Francisco basin (red dashed line) in the São Francisco craton, showing its relationship with neighboring Neoproterozoic fold belts. Inset map shows major cratons in the western Gondwana in a Neoproterozoic paleogeographic configuration: A = Amazonian craton; P = Rio de la Plata craton; K = Kalahari craton; WA = West Africa craton; SFC = São Francisco-Congo craton; PC = Paramirim Corridor. Modified from Reis and Alkmim (Reference Reis and Alkmim2015). (2) Stratigraphic position of the studied section in the Bambui Group columnar section. (3) Geological map of the studied area. The purple dot marks the location of the studied section.
The western portion of the São Francisco craton comprises a succession of siliciclastic and carbonate rocks dated between 1.77 Ga and 0.56 Ga (Pimentel et al., Reference Pimentel, Rodrigues, DellaGiustina, Junges, Matteini and Armstrong2011; Alvarenga et al., Reference Alvarenga, Dardenne, Vieira, Martinho, Guimarães, Santos and Santana2012). The Brasília fold belt that bounds the western margin of the São Francisco craton was deformed during the Brasiliano–Pan African orogeny between 790 Ma and 540 Ma (Pimentel and Fuck, Reference Pimentel and Fuck1992). This fold belt borders to the east with the São Francisco craton covered with undeformed Neoproterozoic strata. It consists of a tectonic domain where only the upper 2 km strata are deformed and a domain further west where both the basement and sediment cover are deformed (Alvarenga et al., Reference Alvarenga, Santos, Vieira, Lima and Mancini2014). A thick interval of Mesoproterozoic and Neoproterozoic sedimentary rocks was deposited along the west portion of the São Francisco craton. These strata are separated into three stratigraphic units, including, in ascending stratigraphic order, the Paranoá Group (Barbosa, Reference Barbosa1963), Jequitaí Formation (Oliveira and Leonardos, Reference Oliveira and Leonardos1943), and Bambuí Group (Rimann, Reference Rimann1917).
The Sete Lagoas Formation, which is the main focus of this work, represents the basal unit of the Bambuí Group and consists of a sequence of carbonate-dominated sediments in the São Francisco basin. Those sediments are characterized by a low total organic carbon (TOC) content of less than 2% (Uhlein et al., Reference Uhlein, Uhlein, Pereira, Caxito, Okubo, Warren and Sial2019; Caetano-Filho et al., Reference Caetano-Filho, Sansjofre, Ader, Paula-Santos, Guacaneme, Babinski, Bedoya-Rueda, Kuchenbecker, Reis and Trindade2021) and relatively low thermal maturity (Reis and Suss, Reference Reis and Suss2016). The δ13Ccarb and δ13Corg excursions indicate a disconnection between the São Francisco basin and the global carbon cycles, which would imply marine isolation and paleogeographic shifts driven by the dynamic changes in marginal orogenic systems (Caetano-Filho et al., Reference Caetano-Filho, Paula-Santos, Guacaneme, Babinski and Bedoya-Rueda2019, Reference Caetano-Filho, Sansjofre, Ader, Paula-Santos, Guacaneme, Babinski, Bedoya-Rueda, Kuchenbecker, Reis and Trindade2021; Guacaneme et al., Reference Guacaneme, Babinski, Bedoya–Rueda, Paula–Santos, Caetano–Filho, Kuchenbecker, Reis and Trindade2021).
The depositional age of the Bambuí Group has long been a matter of debate. The Bambuí Group was initially considered to be Cretaceous (Liais, Reference Liais1872 in Couto et al., Reference Couto, Cordani, Kawashita, Iyer and Moraes1981), but recent studies show that it is probably Ediacaran–Cambrian (Pimentel et al., Reference Pimentel, Rodrigues, DellaGiustina, Junges, Matteini and Armstrong2011; Warren et al., Reference Warren, Quaglio, Riccomini, Simões, Poiré, Strikis, Anelli and Strikis2014; Paula-Santos et al., Reference Paula-Santos, Babinski, Kuchenbecker, Caetano-Filho, Trindade and Pedrosa-Soares2015; Moreira et al., Reference Moreira, Uhlein, Dussin, Uhlein and Misuzaki2020; Sanchez et al., Reference Sanchez, Uhlein and Fairchild2021; DaSilva et al., Reference DaSilva, Pufahl, James, Guimaraes and Reis2022). Geochronological constraints on the Bambuí Group are few and inconclusive. Carbonates of the lower Sete Lagoas Formation yielded Pb–Pb apparent ages of ~740 Ma (Babinski et al., Reference Babinski, Vieira and Trindade2007). However, Caxito et al. (Reference Caxito, Lana, Frei, Uhlein and Sial2021) analyzed samples from crystal-fan-bearing limestone from the base of the Sete Lagoas Formation and obtained U–Pb ages of 615.4 ± 5.9 Ma if both the crystal fans and matrix were considered together, 608.1 ± 5.1 Ma for crystal fans, and 607.2 ± 6.2 Ma for the matrix. The youngest population of detrital zircons from the Sete Lagoas Formation gave U–Pb ages of ~557 Ma (Paula-Santos et al., Reference Paula-Santos, Babinski, Kuchenbecker, Caetano-Filho, Trindade and Pedrosa-Soares2015), and the youngest population of detrital zircons from the Três Marias Formation gave U–Pb ages of ~620 Ma (Rodrigues, Reference Rodrigues2008; Pimentel et al., Reference Pimentel, Rodrigues, DellaGiustina, Junges, Matteini and Armstrong2011), providing maximum age constraints on the host strata. More recently, a zircon U–Pb age of 520.2 ± 5.3 Ma has been reported from a volcanic ash bed in the Serra da Saudade Formation (Moreira et al., Reference Moreira, Uhlein, Dussin, Uhlein and Misuzaki2020), suggesting that the upper Bambuí Group may belong to Stage 2 of the Cambrian System.
The possible occurrence of Cloudina sp. and Corumbella werneri—tubular fossils typically found in terminal Ediacaran rocks—in the lower Sete Lagoas Formation (Warren et al., Reference Warren, Quaglio, Riccomini, Simões, Poiré, Strikis, Anelli and Strikis2014; Perrella Júnior et al., Reference Perrella Júnior, Uhlein, Uhlein, Sial, Pedrosa-Soares and Lima2017) and the putative presence of Treptichnus pedum (Seilacher, Reference Seilacher, Schindewolf and Seilacher1955)—a trace fossil whose first appearance is used to define the base of the Cambrian System—in the Três Marias Formation (Sanchez et al., Reference Sanchez, Uhlein and Fairchild2021) also indicate that perhaps the entire Bambuí Group is Ediacaran–Cambrian, although the conflict with the ~740 Ma Pb–Pb age from the Sete Lagoas Formation (Babinski et al., Reference Babinski, Vieira and Trindade2007) remains unresolved.
Materials and methods
The studied Barreiro section is located in the Santa Luzia quarry near the Barreiro Community, western Januária Municipality, Minas Gerais State, Brazil (Fig. 1). The samples were collected from two different mining benches, as well as exposures in the hills where the Santa Luzia quarry is located. The stratigraphic thickness of the Sete Lagoas Formation in the studied area is about 70 m (Fig. 2).
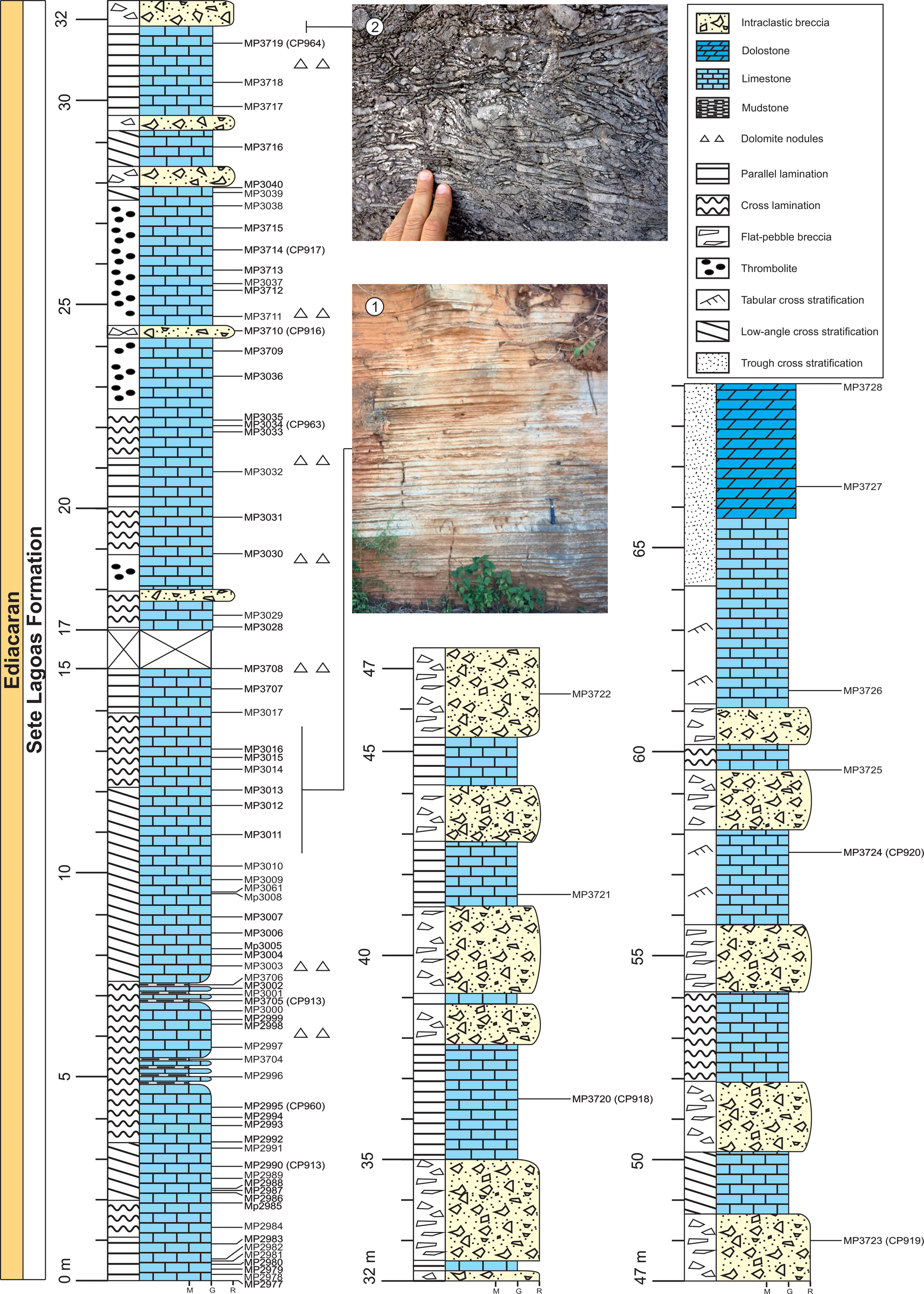
Figure 2. Stratigraphic column and field photographs of the Sete Lagoas Formation at the Barreiro section, Santa Luzia quarry, Januária Municipality, Minas Gerais State, Brazil. (1) Thin-bedded limestone. (2) Intraclastic breccia. Sample horizons are marked with the sample number prefixes MP. Sample numbers in bold mark fossiliferous horizons. The CP- numbers refer to the palynological slides of the illustrated specimens.
The lower 15 m of the studied section consists mainly of dark gray, laminated, microcrystalline lime mudstones with a predominance of parallel bedding with microbial mats. However, there are cross-laminations in layers of fine calcareous grainstones. Microbial mats, silicified ooids, and dolomitic nodules are common at this level (Fig. 2). Intraclastic carbonate breccias, with flat pebbles ranging from <1 to 50 cm and light gray micritic matrix are present at 16 m of the section and above, intercalated with limestones. The top of the section, at around 66 m, is composed of light gray, oolitic, crystalline dolomitic grainstones, sometimes with intraclasts. Such carbonates are cross-stratified. This dolomitic interval presents incipient flat stratification, about 2 cm thick, defined by the changes in the amount of sand-size constituents.
A total of 79 stratigraphic levels were sampled. The curatorship of the rock samples, the remaining organic residues, and the palynological slides followed the protocol presented in Denezine et al. (Reference Denezine, Adôrno, Do Carmo, Guimarães, Walde, Alvarenga, Germs, Antonietto, Gianfranco and Nunes Junior2022). Each residual sample was coded with the MP prefix. All specimens recovered from the Sete Lagoas Formation herein illustrated are coded with the CP prefix. Each illustrated specimen is provided with a slide number followed by England Finder coordinates.
Organic-walled microfossils were extracted from thinly laminated lime mudstones and light- to dark-gray fine-grained limestone samples using acid maceration techniques. The samples were dissolved using hydrochloric and hydrofluoric acids. Residues were rinsed repeatedly in distilled water, and after the residues were settled following each rinse, the supernatant was decanted. No centrifugation was used, to minimize mechanical damage to organic-walled microfossils. No oxidative procedure was applied on organic residues. Transmitted-light photomicrographs were acquired using an Axio Imager.A2 microscope equipped with an AxioCam MRc digital camera (both Carl Zeiss). The organic-walled microfossils were also analyzed using epifluorescence microscopy; however, no fluorescence was observed in the specimens recovered.
Size analysis of Leiosphaeridia specimens is based on the measurement of their vesicle diameters. Vesicle diameter, along with vesicle wall thickness, was used to identify the four morphospecies of Leiosphaeridia present in the Sete Lagoas Formation: Leiosphaeridia crassa (Naumova, Reference Naumova1949), Leiosphaeridia jacutica (Timofeev, Reference Timofeev1966), Leiosphaeridia minutissima (Naumova, Reference Naumova1949), and Leiosphaeridia tenuissima Eisenack, 1958.
Abundance data were collected in this study. All palynological slides were examined thoroughly, and complete specimens were counted. Due to their colonial nature or frequent preservation as fragments, the abundance of Siphonophycus robustum (Schopf, Reference Schopf1968) and Ghoshia januarensis n. sp. was not quantified.
Selected organic-walled microfossils from the Sete Lagoas Formation were analyzed using Raman spectroscopy. Specimens were placed on palynological slides and analyzed on a HORIBA JobinYvon LabRAM HR800 Raman microprobe equipped with a high-resolution 600 mm focal length spectrometer and a 514 nm argon laser source in the Department of Geosciences at Virginia Tech. The laser beam was focused to less than 10 μm in diameter with a 40× objective lens. Raman spectra were acquired using the software Labspec 5.0 with an acquisition time of less than one minute for each analysis and an excitation power of 600 mW.
Raman spectroscopy data were processed using Python modules. Baseline correction was applied to the raw data by adjusting a polynomial (third-order) curve using the Raman data from 800 and 2,100 cm–1 that captures the Raman peaks of carbonaceous material. After baseline correction, the four Raman peaks of carbonaceous material (i.e., D1, D2, D3, D4) were decomposed using fitting G of Kouketsu et al. (Reference Kouketsu, Mizukami, Mori, Endo, Aoya, Hara, Nakamura and Wallis2014).
The processed Raman data were subjected to principal component analysis (PCA) in Python. The peak position, peak height, and full width at half maximum of the four Raman peaks of carbonaceous material (D1–D4) were used in PCA. The Python package for PCA is publicly available (Mazoni, Reference Mazoni2021), and PCA in this study used the Python modules Numpy (Harris et al., Reference Harris, Millman, van der Walt, Gommers and Virtanen2020), Scipy (Virtanen et al., Reference Virtanen, Gommers, Oliphant, Haberland and Reddy2020), and Rampy (Le Rosq, Reference Le Rosq2021).
Repository and institutional abbreviation
Types, figured specimens, and other specimens examined in this study are deposited in the Paleontological Collection under the prefix MP in the Museum of Geosciences (MGeo-UnB), University of Brasília, Brasília, Brazil.
Systematic paleontology
The suprageneric taxonomy follows the system of modern cyanobacteria and the informal classification of acritarchs (e.g., Butterfield et al., Reference Butterfield, Knoll and Swett1994; Sergeev and Schopf, Reference Sergeev and Schopf2010). Seven organic-walled microfossil species were recovered: Ghoshia januarensis n. sp., Germinosphaera bispinosa Mikhailova, Reference Mikhailova and Sokolov1986, Leiosphaeridia crassa (Naumova, Reference Naumova1949), Leiosphaeridia jacutica (Timofeev, Reference Timofeev1966), Leiosphaeridia minutissima (Naumova, Reference Naumova1949), Leiosphaeridia tenuissima Eisenack, 1958, and Siphonophycus robustum (Schopf, Reference Schopf1968) (Figs. 3, 4). Two of them, Siphonophycus robustum and Ghoshia januarensis, are considered cyanobacteria. Four of them, Leiosphaeridia crassa, Leiosphaeridia jacutica, Leiosphaeridia minutissima, and Leiosphaeridia tenuissima, are sphaeromorph acritarchs traditionally regarded as protists. The phylogenetic affinity of Germinosphaera bispinosa is uncertain.
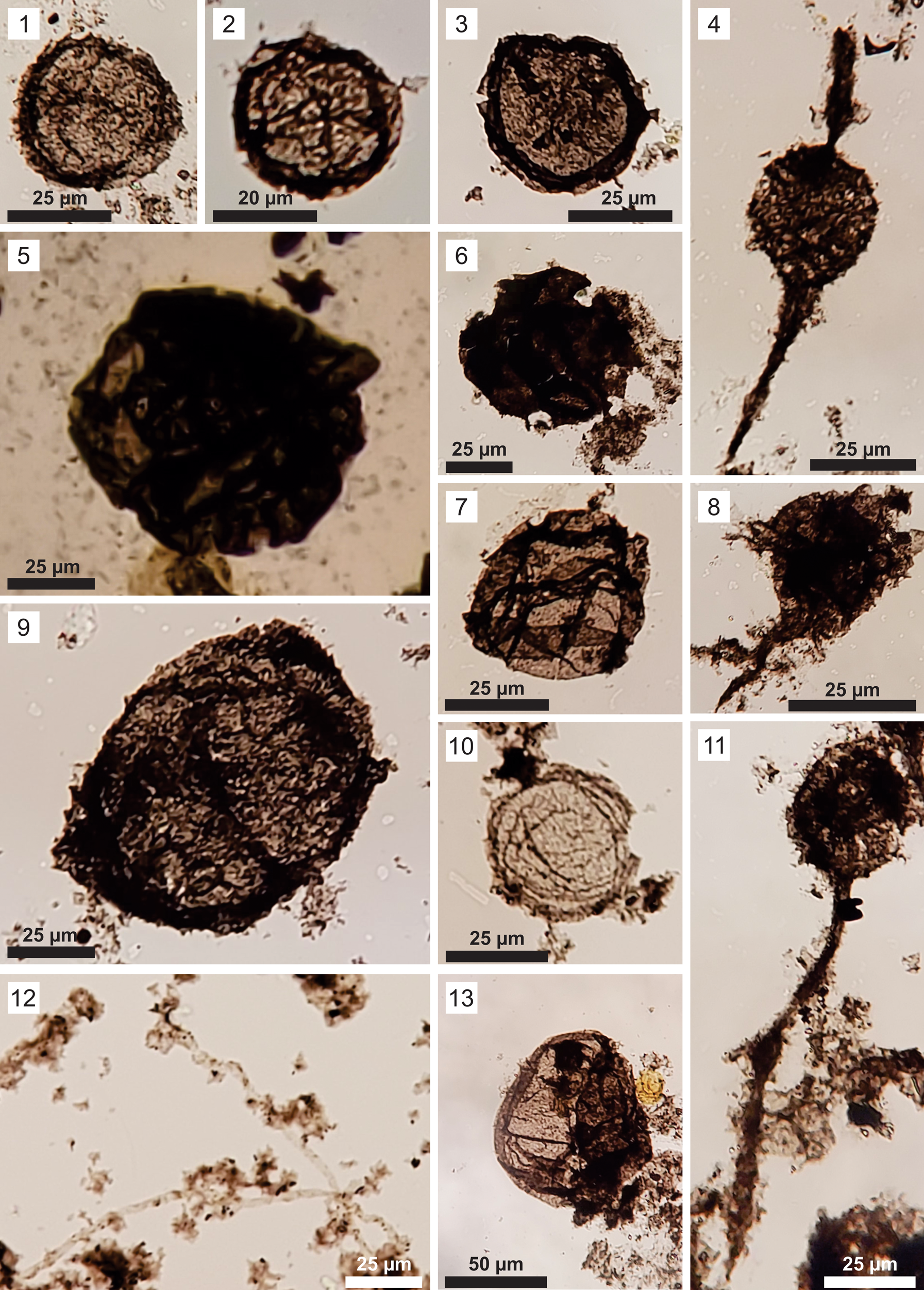
Figure 3. Organic-walled microfossils from the Sete Lagoas Formation at the Barreiro section. Slide number and England Finder coordinates (in parentheses) are given for each illustrated specimen. (1–3, 7, 10) Leiosphaeridia minutissima: (1) CP962 (S32); (2) CP962 (F48); (3) CP918 (K22); (7) CP964 (P29); (10) CP963 (F33). (4, 8, 11) Germinosphaera bispinosa, all in slide CP917 (EF coordinates: S26, I43, and O28, respectively). (5) Leiosphaeridia jacutica, CP913 (Y23). (6) Leiosphaeridia crassa, CP964 (H29). (9, 13) Leiosphaeridia tenuissima, all in slide CP914 (EF coordinates: Q30 and R23, respectively). (12) Siphonophycus robustum, CP960 (I50).
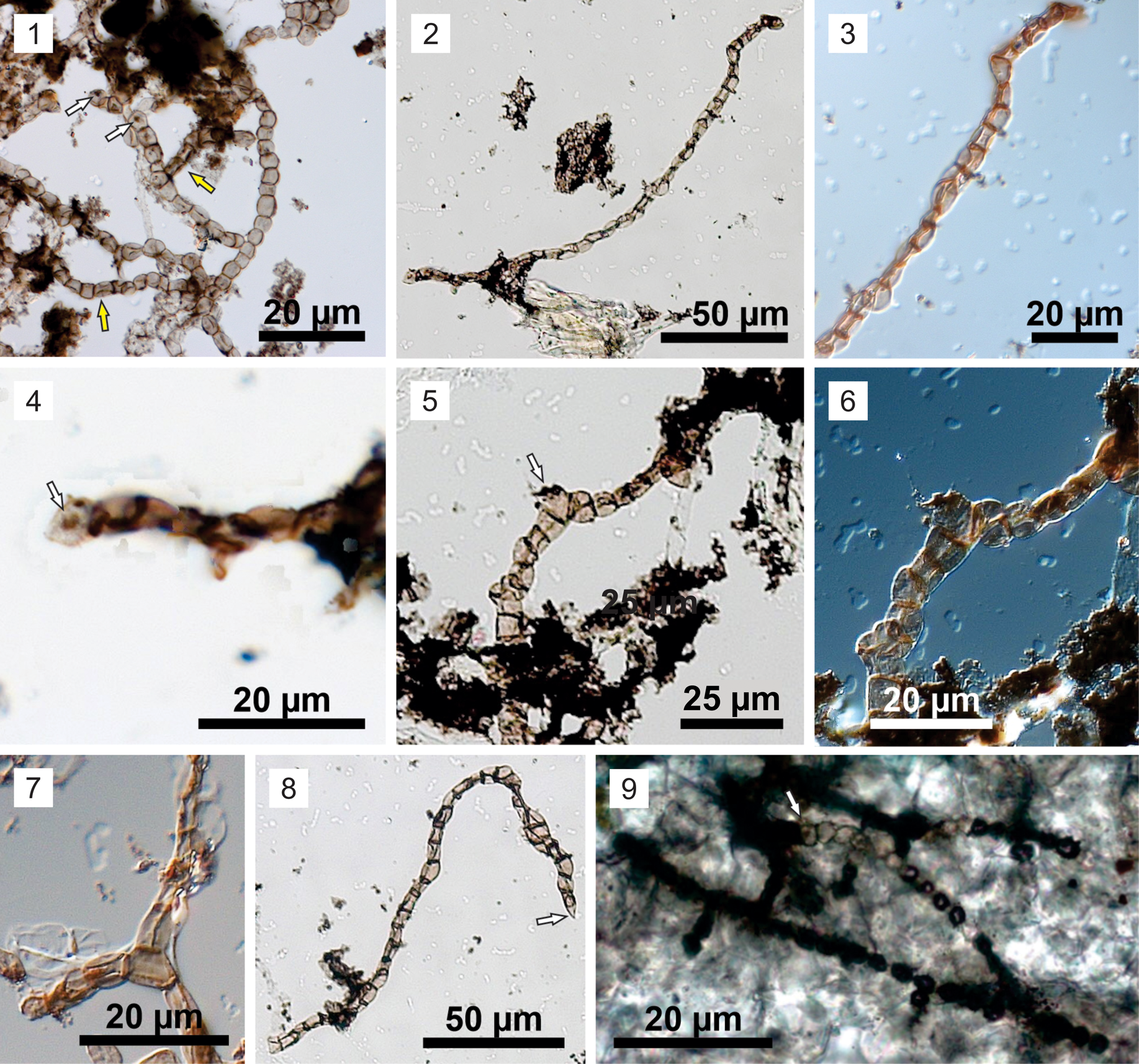
Figure 4. Ghoshia januarensis n. sp. from the Sete Lagoas Formation in the Barreiro section. (1) Holotype: CP916 (E46). Note dark spots inside cells indicated by white arrows. Yellow arrows indicate slightly deflated and deformed cells. (2–8) Paratypes: (2–4) CP919 (E18); (3) magnified view of the upper right part of (2), showing slightly deflated and deformed cells; (4) magnified view of the lower left part of (2), showing dark spot in terminal cell (arrow); (5, 6) CP919 (J16); (6) dark-field view of the central part of (5), showing a polyhedral cell (arrow in 5). (7) CP919 (J26); note polyhedral cell at branching point. (8) CP920 (N18/3), showing pointed terminal cell (arrow). (9) Specimen identified in a petrographic thin section of the Sete Lagoas Formation at the Barreiro section in the Januária area. Reproduced from Perrella Júnior et al. (Reference Perrella Júnior, Uhlein, Uhlein, Sial, Pedrosa-Soares and Lima2017) with permission.
Kingdom Eubacteria Woese and Fox, Reference Woese and Fox1977
Phylum Cyanobacteria Stanier et al., Reference Stanier, Sistrom, Hansen, Whitton and Castenholz1978
Class Hormogoneae Thuret, Reference Thuret1875
Order Oscillatoriales Elenkin, Reference Elenkin1949
Family Oscillatoriaceae Kirchner, Reference Kirchner, Engler and Prantl1900
Genus Siphonophycus Schopf, Reference Schopf1968
Type species
Siphonophycus kestron Schopf, Reference Schopf1968 (holotype: Paleobotany Collection Harvard University no. 58469, stage coordinates 33.6 × 101.4) from the black chert facies in the middle third of the late Precambrian Bitter Springs Formation, exposed on the south slope of the ridge about 1 mile north of Ross River Tourist Camp (Love's Creek Homestead), 40 miles northeast of Alice Springs, Northern Territory, Australia, by original designation.
Other species
Siphonophycus thulenema Butterfield in Butterfield et al., Reference Butterfield, Knoll and Swett1994; Siphonophycus septatum (Schopf, Reference Schopf1968); Siphonophycus robustum (Schopf, Reference Schopf1968); Siphonophycus typicum (Hermann, Reference Hermann and Timfeev1974); Siphonophycus kestron Schopf, Reference Schopf1968; Siphonophycus solidum (Golub, Reference Golub and Sokolov1979); Siphonophycus punctatum Maithy, Reference Maithy1975; and Siphonophycus gigas Tang et al., Reference Tang, Pang, Xiao, Yuan, Ou and Wan2013.
Original diagnosis by Schopf (Reference Schopf1968)
“Thallus broad, tubular, nonseptate, unbranched, commonly quite long, finely rugose in surface texture. Thallus cylindrical, somewhat tapered toward apices, solitary, straight to slightly bent, up to 180 μm long (incomplete specimen), occasionally folded and distorted. Apices apparently capitate, more-or-less constricted adjacent to expanded, broadly conical, bluntly pointed terminus. Thallus quite broad, 8.3–15.00 μm wide, commonly about 12.5 μm wide (based on five specimens), ornamented and ringed by finely punctate surficial ridges regularly spaced out 2/3 μm apart. Reproductive structures unknown.”
Emended diagnosis by Knoll et al. (Reference Knoll, Swett and Mark1991)
“Tubular, filamentous microfossils, nonseptate and unbranched, with little or no tapering toward filament termini; tubes truncated and open at ends or with closed, more or less hemispherical terminations; walls typically preserved as chagrenate to finely reticulate organic matter, but may be preserved as carbonate rinds.”
Remarks
The genus Siphonophycus is characterized by smooth and thin wall filaments without ornamentation. The taxon is traditionally interpreted as representing empty sheaths of filamentous cyanobacteria, but because of simple morphology, it could include a range of bacterial and eukaryotic organisms (Butterfield et al., Reference Butterfield, Knoll and Swett1994). Although it is here placed under cyanobacteria, we recognize that Siphonophycus is a form taxon, and several other genera of filamentous microfossils (e.g., Eomycetopsis, Tenuofilum, and Leiotrichoides) are regarded as synonyms of Siphonophycus (Knoll et al., Reference Knoll, Swett and Mark1991).
Siphonophycus robustum (Schopf, Reference Schopf1968) emend. Knoll et al., Reference Knoll, Swett and Mark1991
Figure 3.12
- Reference Schopf1968
Eomycetopsis robusta Schopf, p. 685, pl. 82, figs. 2, 3, pl. 83, figs. 1–4.
- Reference Schopf1968
Eomycetopsis filiformis Schopf, p. 685, pl. 82, figs. 1, 4, pl. 83, figs. 5–8.
- Reference Knoll and Golubic1979
Eomycetopsis robusta; Knoll and Golubic, p.149, fig. 4a, b.
- Reference Mendelson and Schopf1982
Eomycetopsis robusta; Mendelson and Schopf, p. 59, pl. 1, figs. 9, 10.
- Reference Sergeev1984
Eomycetopsis robusta; Sergeev, p. 436, fig. 2a–ã.
- Reference Hofmann and Jackson1991
Eomycetopsis robusta; Hofmann and Jackson, p. 367, fig. 5.1–5.3, 5.8.
- Reference Knoll, Swett and Mark1991
Siphonophycus robustum (Schopf, Reference Schopf1968); Knoll et al., p. 565, fig. 10.3, 10.5.
- Reference Zang and Walter1992
Eomycetopsis robusta; Zang and Walter, p. 314, pl. 17, figs. g–I, p. 308, pl. 18, fig. g.
- Reference Sergeev1992
Eomycetopsis robusta; Sergeev, p. 93, pl. 7, figs. 9, 10, pl. 16, figs. 3, 6, 7, 10; pl. 19, figs. 1, 5–10, pl. 24, fig. 7.
- Reference Golovenok and Belova.1993
Eomycetopsis robusta; Golovenok and Belova, pl. 2, fig. å.
- Reference Butterfield, Knoll and Swett1994
Siphonophycus robustum; Butterfield et al., p. 64, fig. 26a, g.
- Reference Hofmann and Jackson1994
Siphonophycus robustum; Hofmann and Jackson, p. 10, fig. 11.5.
- Reference Sergeev1994
Siphonophycus robustum; Sergeev, p. 250, fig. 8f.
- Reference Sergeev, Knoll, Kolosova and Kolosov.1994
Siphonophycus robustum; Sergeev et al., pl. 3, fig. 6.
- Reference Kumar and Srivastava1995
Siphonophycus robustum; Kumar and Srivastava, p. 114, fig. 14c–e.
- Reference Zang1995
Siphonophycus robustum; Zang, p. 172, figs. 26a, 32l, m.
- Reference Sergeev, Knoll and Petrov1997
Siphonophycus robustum; Sergeev et al., p. 230, fig. 14a.
- Reference Kumar and Venkatachala1998
Siphonophycus robustum; Kumar and Venkatachala, p. 63, fig. 6c.
- Reference Sergeev2001
Siphonophycus robustum; Sergeev, p. 442, fig. 7.8, 7.9.
- Reference Sergeev and Lee2001
Siphonophycus robustum; Sergeev and Lee, p. 6, pl. 1, figs. 1, 2, 7, 11, 12.
- Reference Samuelsson and Butterfield2001
Siphonophycus robustum; Samuelsson and Butterfield, p. 240, figs. 2b, 9h.
- Reference Gaucher, Boggiani, Sprechmann, Sial and Fairchild2003
Siphonophycus robustum; Gaucher et al., fig. 6c, d.
- Reference Gaucher and Germs2003
Siphonophycus robustum; Gaucher and Germs, fig. 7.12.
- Reference Sharma and Sergeev2004
Siphonophycus robustum; Sharma and Sergeev, figs. 3c, 4a, 6b, e, 7c, f, 9e, 11f.
- Reference Sergeev and Lee2004
Siphonophycus robustum; Sergeev and Lee, pl. 2, fig. 4.
- Reference Tiwari and Pant2004
Siphonophycus robustum; Tiwari and Pant, fig. 3i, n.
- Reference Prasad, Uniyal and Asher2005
Siphonophycus robustum; Prasad et al., pl. 1, fig. 7, pl. 5, fig. 12.
- Reference Sergeev2006
Siphonophycus robustum; Sergeev, p. 213, pl. 6, figs. 9, 10, pl. 17, fig. 1, pl. 19, figs. 8, 9, pl. 22, figs. 1, 2, 7, 8, 11, 12, pl. 25, figs. 1, 3, pl. 27, figs. 4, 5, pl. 28, fig. 2, pl. 36, figs. 1, 2, pl. 44, figs. 1–7, 13, pl. 46, figs. 7–10, pl. 48, fig. 4.
- Reference Kumar and Pandey2008
Siphonophycus robustum; Kumar and Pandey, fig. 3a, b.
- Reference Sergeev, Sharma and Shukla2008
Siphonophycus robustum; Sergeev et al., pl. 6, figs. 1, 5, 6, pl. 9, figs. 1–3, 5–7.
- Reference Tiwari and Pant2009
Siphonophycus robustum; Tiwari and Pant, fig. 6a–c.
- Reference Dong, Xiao, Shen, Zhou, Li and Yao2009
Siphonophycus robustum; Dong et al., p. 30, fig. 6.12.
- Reference Sergeev and Schopf2010
Siphonophycus robustum; Sergeev and Schopf, p. 387, fig. 6.4.
- Reference Sergeev, Sharma and Shukla2012
Siphonophycus robustum; Sergeev et al., p. 309, pl. 21, figs. 2, 4, 8–10.
- Reference Pandey and Kumar2013
Siphonophycus robustum; Pandey and Kumar, p. 504, fig. 4e.
- Reference Knoll, Wörndle and Kah2013
Siphonophycus robustum; Knoll et al., fig. 4c.
- Reference Tang, Pang, Xiao, Yuan, Ou and Wan2013
Siphonophycus robustum; Tang et al., fig. 13b, m.
- Reference Babu, Singh and Mehrotra2014
Siphonophycus robustum; Babu et al., fig. 3q.
- Reference Liu, Xiao, Yin, Chen, Zhou and Li2014
Siphonophycus robustum; Liu et al., fig. 110.1.
- Reference Vorob'eva, Sergeev and Petrov2015
Siphonophycus robustum; Vorob'eva et al., fig. 9.14.
- Reference Tang, Pang, Yuan, Wan and Xiao2015
Siphonophycus robustum; Tang et al., fig. 18c.
- Reference Schopf, Sergeev and Kudryavtsev2015
Siphonophycus robustum; Schopf et al., p. 716, fig. 11.11.
- Reference Porter and Riedman2016
Siphonophycus robustum; Porter and Riedman, p. 837, fig. 16.4.
- Reference Sergeev, Knoll, Vorob'eva and Sergeeva2016
Siphonophycus robustum; Sergeev et al., fig. 8.4.
- Reference Baludikay, Storme, François, Baudet and Javaux2016
Siphonophycus robustum; Baludikay et al., fig. 11n.
- Reference Tang, Hughes, McKenzie, Myrow and Xiao2017
Siphonophycus robustum; Tang et al., fig. 8a, c, d.
- Reference Shi, Feng, Khan and Zhu2017a
Siphonophycus robustum; Shi et al., fig. 6.3, 6.5.
- Reference Shi, Feng, Khan, Awramik and Zhu2017b
Siphonophycus robustum; Shi et al., p. 721, fig. 3e, f.
- Reference Javaux and Knoll2017
Siphonophycus robustum; Javaux and Knoll, p. 212, fig. 5.11.
- Reference Beghin, Storme, Blanpied, Gueneli, Brocks, Poulton and Javaux2017
Siphonophycus robustum; Beghin et al., pl. 3, fig. i.
- Reference Sergeev, Vorob'eva, Petrov and Semikhatov2017b
Siphonophycus robustum; Sergeev et al., p. 290, fig. 5.10, 5.11.
- Reference Li, Pang, Chen, Zhou, Han, Yang, Wang, Yang and Yin2019
Siphonophycus robustum; Li et al., fig. 15h.
- Reference Loron, Rainbird, Turner, Greenman and Javaux2019
Siphonophycus robustum; Loron et al., fig. 3f.
- Reference Arrouy, Gaucher, Poiré, Xiao, Peral, Warren, Bykova and Quaglio2019
Siphonophycus robustum; Arrouy et al., fig. 6f.
- Reference Knoll, Germs, Tankard and Welsink2020
Siphonophycus robustum; Knoll et al., p. 6, fig. 3n, o.
- Reference Arvestål and Willman2020
Siphonophycus robustum; Arvestål and Willman, p. 22, fig. 10f.
- Reference Shukla, Sharma and Sergeev2020
Siphonophycus robustum; Shukla et al., p. 496, fig. 5e.
- Reference Miao, Moczydłowska and Zhu2021
Siphonophycus robustum; Miao et al., p. 17, fig. 9e.
- Reference Denezine, Adôrno, Do Carmo, Guimarães, Walde, Alvarenga, Germs, Antonietto, Gianfranco and Nunes Junior2022
Siphonophycus robustum; Denezine et al., fig. 11.6.
For additional synonyms, see Butterfield et al. (Reference Butterfield, Knoll and Swett1994).
Holotype
Paleobotanical collections, Harvard University (thin section Bit. Spr. 10-1, number 58491), from Neoproterozoic Bitter Springs Formation, Amadeus Basin, Australia (Schopf, Reference Schopf1968, pl. 83, fig. 1).
Original diagnosis by Schopf (Reference Schopf1968)
“Filaments commonly solitary, occasionally in groups of a few entangled filaments, rarely showing plectenchymatous organization. Lateral walls approximately1/3–3/4 μ thick, markedly coriaceous, coarsely and irregularly granular in surface texture. Filaments up to 135 μ long (incomplete filament), more-or-less regularly cylindrical with a variance in diameter of less than 0.8 μ from the widest to the most narrow portion of the filament; 2.8–4.2 μ in diameter with an average width (20 filaments measured of 3.5 μ). Septate portions of filament vary in length, commonly less than 25 μ long, with filaments commonly constricted or overlapping at the septa; overlapping portions commonly with rounded ends. Reproductive structures unknown.”
Emended diagnosis by Knoll and Golubic (Reference Knoll and Golubic1979)
“Filaments cylindrical; unbranched; tubular (nonseptate); bent, sinuous and tortuous; partially flattened, circular to elliptical in cross section; intertwined to form more or less dense meshworks; long. Surface coarsely to irregularly granular in texture. Occasional cylindrical and evenly spaced inclusions, homogeneously filled with fine-grained carbonaceous matter and centrally located in the ‘bore’ of the tube. Filaments tubular with average diameters expressed as mean ± standard deviation 2.95 ±μm (range 2.0–4.4, n = 60). Occasional long cylindrical inclusions, 1.09 ± 0.36 μm (n = 8) in diameter, 3–4 μm long located centrally within tubular filaments.”
Emended diagnosis by Knoll et al. (Reference Knoll, Germs, Tankard and Welsink2020)
“A species of Siphonophycus with tubes 2–4 μm in cross-sectional diameter.”
Occurrence in the studied section
MP2985, MP2995, MP3040, MP3708, MP3709, and MP3710.
Illustrated specimen
CP960 (3 μm in diameter).
Remarks
Filamentous microfossils from the Sete Lagoas Formation are scarce and restricted to Siphonophycus robustum (Schopf, Reference Schopf1968).
Order Stigonematales Geitler, Reference Geitler1925
Family Capsosiracea Geitler, Reference Geitler1925
Genus Ghoshia Mandal and Maithy in Mandal et al., Reference Mandal, Maithy, Barman and Verma1984
Type species
Ghoshia bifurcata Mandal and Maithy in Mandal et al., Reference Mandal, Maithy, Barman and Verma1984.
Original diagnosis presented by Mandal and Maithy in Mandal et al. (Reference Mandal, Maithy, Barman and Verma1984)
“Thallus heterotrichous, erect filaments arising from basal horizontally creeping thallus, densely packed, truly laterally branched, with cells in one or two series; sheath absent; reproduction not observed.”
Ghoshia januarensis new species
Figure 4
- Reference Perrella Júnior, Uhlein, Uhlein, Sial, Pedrosa-Soares and Lima2017
Fossil filaments consisting of aligned rounded cells, Perrella Júnior et al., p. 138. fig. 7h.
- Reference Denezine, Adôrno, Do Carmo, Guimarães, Walde, Alvarenga, Germs, Antonietto, Gianfranco and Nunes Junior2022
Goshia sp.; Denezine et al., fig. 11.5.
Type specimens
Holotype: CP916. Paratypes: CP919 and CP920. Specimens are housed in the Research Collection, Museum of Geosciences, Institute of Geosciences, University of Brasília, Federal District, Brazil.
Type locality
Sete Lagoas Formation, Bambuí Group, Santa Luzia quarry, Municipality of Januária, Minas Gerais State, Brazil.
Type horizon
Intraclastic breccia from the Sete Lagoas Formation, Bambuí Group. Stratigraphic level: between 31.5 and 36.4 m.
Diagnosis
A species of Ghoshia characterized by spherical to doliform cells that are 3–10 μm in diameter. Cells are organized to form uniserial chains that branch irregularly.
Occurrence in the studied section
MP2980, MP3013, MP3015, MP3040, MP3710, MP3714, MP3718, MP3723, and MP3724.
Description
Uniserial cell chains that branch irregularly. Cells are spherical (Fig. 4.1), doliform (Fig. 4.2–4.6, 4.9), or polyhedral (Fig. 4.7), with smooth cell walls. Side branches arise more or less perpendicularly to the main branches. Cells at the branching points are often polyhedral (Fig. 4.7). Cells 3–10 μm in diameter. Deformation folds, likely resulting from compression, are present in some cells (Fig. 4.2, 4.7, 4.8).
Etymology
In reference to the Municipality of Januária, Minas Gerais State, Brazil.
Illustrated specimens
CP916, CP919, and CP920.
Remarks
The Sete Lagoas specimens are somewhat similar to Arctacellularia German in Timofeev et al., Reference Timofeev, Hermann and Mikhailova1976 in their uniserial filaments consisting of spherical, doliform, and polyhedral cells. However, unlike the Sete Lagoas specimens, Arctacellularia does not branch. The Sete Lagoas specimens are also similar to the Devonian cyanobacteria Langiella Croft and George, Reference Croft and George1959, Kidstoniella Croft and George, Reference Croft and George1959, and Rhyniella Croft and George, Reference Croft and George1959 in having branching filaments. However, these Devonian genera can be distinguished by the presence of morphologically differentiated heterocysts and akinetes or by the presence of a sheath (Croft and George, Reference Croft and George1959). The Sete Lagoas specimens are best placed in the genus Ghoshia, which is characterized by branching uniserial filaments consisting of largely undifferentiated cells. The new species proposed here, Ghoshia januarensis, resembles Ghoshia bifurcata Mandal and Maithy in Mandal et al., Reference Mandal, Maithy, Barman and Verma1984 in cell size but differs in its more variable cell shape; Ghoshia januarensis has spherical, doliform, and polyhedral cells, whereas Ghoshia bifurcata is said to have “drum-shaped to rectangular” cells (Mandal et al., Reference Mandal, Maithy, Barman and Verma1984). In addition, some specimens of Ghoshia bifurcata (including the holotype; Mandal et al., Reference Mandal, Maithy, Barman and Verma1984, pl. 4, fig. 30) seem to have cell aggregates that are not uniserially organized.
A specimen from the Sete Lagoas Formation in the Januária area illustrated as “fossil filaments consisting of aligned rounded cells” (Perrella Júnior et al., Reference Perrella Júnior, Uhlein, Uhlein, Sial, Pedrosa-Soares and Lima2017, fig. 7H) shares the same characteristics of Ghoshia januarensis, including uniserial and branching filaments consisting of spherical cells. Thus, this specimen is here identified as Ghoshia januarensis. It is important to point out that the specimen illustrated in Perrella Júnior et al. (Reference Perrella Júnior, Uhlein, Uhlein, Sial, Pedrosa-Soares and Lima2017) was observed in a petrographic thin section, ruling out the possibility of modern contamination.
Raman spectra of the analyzed microfossils (Fig. 5) display well-developed D1 and D2 bands positioned at 1,350 cm–1 and 1,620 cm–1, respectively. These characteristics are typical of organic matter spectra (Kouketsu et al., Reference Kouketsu, Mizukami, Mori, Endo, Aoya, Hara, Nakamura and Wallis2014). The Raman data show that the four analyzed specimens of Ghoshia januarensis, including the holotype, are distinct from other organic-walled microfossils from the Sete Lagoas Formation (Fig. 5). Relative to other organic-walled microfossils from the Sete Lagoas Formation, Ghoshia januarensis specimens exhibit broader peaks (Fig. 5.1). PCA analysis of Raman parameters also shows that Ghoshia januarensis specimens are separate from other organic-walled microfossils from the Sete Lagoas Formation (Fig. 5.2). Therefore, it is possible that specimens of Ghoshia januarensis have different thermal history from other organic-walled microfossils in the Sete Lagoas Formation, indicating that the former could be contaminations. However, the three specimens of Ghoshia januarensis that were analyzed for Raman spectroscopy in Fig. 5.2 overlap with the other organic-walled microfossils largely along the second PCA axis; the difference is mainly along the primary PCA axis. A similar situation is found in the four specimens of Leiosphaeridia minutissima that were analyzed for Raman spectroscopy (labeled as A, B, G, and H in Fig. 5.2): they exhibit a limited range and overlap with other organic-walled microfossils from the Sete Lagoas Formation along the primary PCA axis, but two specimens (labeled A and B in Fig. 5.2) are distinct from all other specimens along the secondary PCA axis. Although subtle differences in carbonaceous material Raman characteristics could be taken as evidence for different degrees of thermal maturation (Kouketsu et al., Reference Kouketsu, Mizukami, Mori, Endo, Aoya, Hara, Nakamura and Wallis2014), recent studies show that such differences can result from differences in organic precursors (Qu et al., Reference Qu, Engdahl, Zhu, Vajda and McLoughlin2015; Pang et al., Reference Pang, Tang, Wu, Li, Chen, Wan, Yuan, Bodnar and Xiao2020). Considering that Ghoshia januarensis specimens showed no fluorescence as would modern organic contaminations, and that Ghoshia januarensis has been found in a petrographic thin section of the Sete Lagoas Formation (Perrella Júnior et al., Reference Perrella Júnior, Uhlein, Uhlein, Sial, Pedrosa-Soares and Lima2017), we conclude that Ghoshia januarensis is indigenous to the Sete Lagoas Formation.
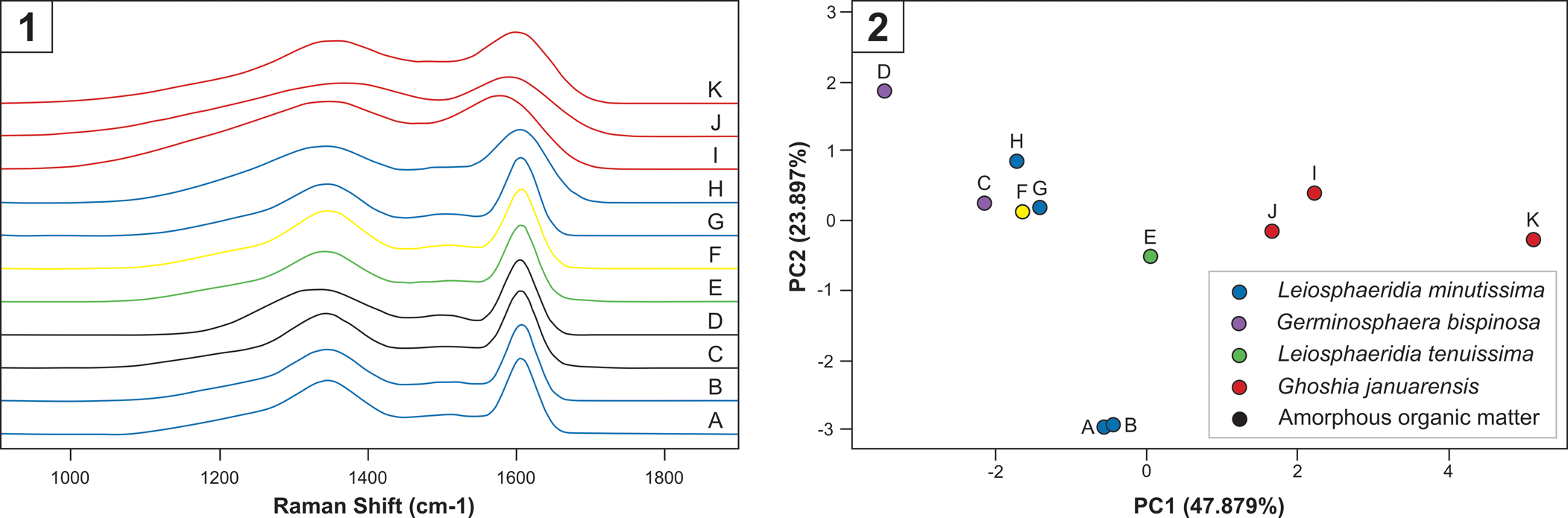
Figure 5. Raman spectroscopic data of organic-walled microfossils and amorphous organic matter from the Sete Lagoas Formation at the Barreiro section. (1) Baseline-corrected and fitted Raman spectra. Legends are shown in Figure 5.2. Note that Raman spectra of Ghoshia januarensis (J from holotype and I, K from paratypes) have broader peaks of carbonaceous matter around 1,350 cm–1 and 1,600 cm–1 relative to other Sete Lagoas organic-walled microfossils. (2) Principal component analysis of deconvolved Raman data. Samples: A–B and J, CP916; C–D, CP917; E, I, K, CP920; F, H, MP3728; G, MP3723.
Group Acritarcha Evitt, Reference Evitt1963
Subgroup Sphaeromorphitae Downie et al., Reference Downie, Evitt and Sarjeant1963
Genus Leiosphaeridia Eisenack, 1958
Type species
Leiosphaeridia baltica Eisenack, 1958 (in Eisenack, Reference Eisenack1958b).
Other species
Fensome et al. (Reference Fensome, Williams, Bars, Freeman and Hill1990) revised all Leiosphaeridia species and listed 167 valid species.
Original diagnosis presented by Eisenack (Reference Eisenack1958b) in German
“Hohlkugelförmige, dünnwandige und aus einer sehr widerstandsfähigen, hellgelb bis dunkelrotbraun durchscheinenden organischen Substanz bestehende Organismenreste, die oft in scheibenförmig zusammengepreßtem Zustande oder auch unregelmäßig verfaltet überliefert sein können. Wand, auch in erwachsenem Zustande, stets ohne Wandporen (Unterschied zu Tasmanites). Pylome vorhanden.”
Translation of original diagnosis presented by Eisenack (Reference Eisenack1958b)
Hollow spherical, thin-walled organism residues consisting of a very resistant, light yellow to dark red-brown translucent, organic substance, which can often be preserved as a disc-shaped compressed state or an irregularly folded structure. Wall, even when fully grown, always without wall pores (in contrast to Tasmanites). Pylome present.
Emended diagnosis by Downie and Sarjeant (Reference Downie and Sarjeant1963)
“Spherical to ellipsoidal bodies without processes, often collapsed or folded, with or without pylomes. Walls granular, punctate or unornamented, thin. Without divisions into fields and without transverse or longitudinal furrows or girdles.”
Emended diagnosis by Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) in Russian
“Сфероидальные оболочки с гладкой, точеечной или зеррнистой поверхиостью размером от 2–3 до 750 мкм. Толщина стенки от долей микрометра до 3–10 мкм. В ископаемом состоянии сплющены и осложнены складками смятия различной формы и размеров.”
Translation of emended diagnosis by Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989)
Spheroidal vesicle with a smooth, punctate, or granular surface ranging in size from 2–3 to 750 μm. The wall thickness varies from fractions of a micrometer to 3–10 μm. The specimens are flattened and can have compressional folds of various shapes and sizes.
Remarks
A great number of species of the genus Leiosphaeridia have been reported from the Proterozoic, and many of them have very long stratigraphic ranges, e.g., from the Paleoproterozoic to the Mesozoic (Lamb et al., Reference Lamb, Awramik, Chapman and Zhu2009). There are even reports of Leiosphaeridia species from the Miocene (Hannah et al., Reference Hannah, Wilson and Wrenn2000). Because of its simple morphologies, the genus Leiosphaeridia is regarded as a form taxon with diverse phylogenetic affinities, and it is classified in the Acritarcha (Jankauskas et al., Reference Jankauskas, Mikhailova and German1989; Grey, Reference Grey2005; Sergeev and Schopf, Reference Sergeev and Schopf2010), although Sergeev and Schopf (Reference Sergeev and Schopf2010) consider this taxon as belonging to the Kingdom Protista, a proposition followed here. It is important to point out that some authors relate Leiosphaeridia species to chlorophyceaens (Moczydlowska et al., Reference Moczydlowska, Landing, Zang and Palacios2011; Moczydłowska, Reference Moczydłowska2016). Downie and Sarjeant (Reference Downie and Sarjeant1963) emended the diagnosis of the genus Leiosphaeridia to exclude the reference of the vesicle color since it could reflect diagenetic features. Moreover, the maceration protocol could affect the color of organic vesicles due to the use of oxidizing solutions. Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) specified that the diameter of the vesicle of Leisophaeridia species ranges from 2–3 to 750 μm. Furthermore, Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) divided the smooth-walled Leiosphaeridia species into four species according to vesicle diameter and wall thickness, a form-taxonomical scheme followed in the present work. Butterfield et al. (Reference Butterfield, Knoll and Swett1994) suggested that Leiosphaeridia should be restricted to spheroidal fossils with vesicle walls less than 2 μm thick so that it can be differentiated from Chuaria circularis Walcott, Reference Walcott1899, which has thicker vesicle walls (2–3 μm single-wall thickness).
Leiosphaeridia crassa (Naumova, Reference Naumova1949) Jankauskas in Jankauskas et al., Reference Jankauskas, Mikhailova and German1989
Figure 3.6
- Reference Naumova1949
Leiotriletes crassus Naumova, p. 54, pl. 1, figs. 5, 6, pl. 2, figs. 5, 6.
- Reference Pykhova1973
Leiopsophosphaera crassa; Pykhova, p. 99, pl. 2, fig. 3.
- Reference Jankauskas, Mikhailova and German1989
Leiosphaeridia crassa (Naumova, Reference Naumova1949) Jankauskas in Jankauskas et al., p. 75, pl. 9, figs. 5–10.
- Reference Zang and Walter1992
Leiosphaeridia crassa; Zang and Walter, p. 289, pl. 9, figs. a–k, pl. 12, fig. k, pl. 14, figs. e, h.
- Reference Butterfield, Knoll and Swett1994
Leiosphaeridia crassa; Butterfield et al., p. 40, figs. 16f, 23k.
- Reference Hofmann and Jackson1994
Leiosphaeridia crassa; Hofmann and Jackson, p. 22, fig. 1.19–1.29.
- Reference Knoll1994
Leiosphaeridia crassa; Knoll, fig. 4b.
- Reference Zang1995
Leiosphaeridia crassa; Zang, p. 166, figs. 21d, 28c, d.
- Reference Yin and Guan1999
Leiosphaeridia crassa; Yin and Guan, p. 131, figs. 3.8, 4.5, 5.3, 5.5, 5.7, 5.11, 6.2–6.6, 6.9, 6.12.
- Reference Javaux, Knoll and Walter2004
Leiosphaeridia crassa; Javaux et al., fig. 4e–i.
- Reference Sergeev and Lee2004
Leiosphaeridia crassa; Sergeev and Lee, p. 21, pl. 3, figs. 4, 5.
- Reference Tiwari and Pant2004
Leiosphaeridia crassa; Tiwari and Pant, p. 1736, fig. 3v.
- Reference Grey2005
Leiosphaeridia crassa; Grey, p. 179, figs. 63a–c, 64a–d.
- Reference Marshall, Javaux, Knoll and Walter2005
Leiosphaeridia crassa; Marshall et al., fig. 1e.
- Reference Prasad, Uniyal and Asher2005
Leiosphaeridia crassa; Prasad et al., pl. 1, figs. 1, 2, pl. 4, fig. 16, pl. 5, fig. 18, pl. 9, figs. 10, 11.
- Reference Javaux and Marshal2006
Leiosphaeridia crassa; Javaux and Marshal, fig. 3.4–3.6.
- Reference Sergeev and Seong-Joo2006
Leiosphaeridia crassa; Sergeev and Seong-Joo, p. 15, pl. 2, figs. 2a–c, 5.
- Reference Moczydłowska2008a
Leiosphaeridia crassa; Moczydłowska, p. 84, figs. 7a, 8g.
- Reference Moczydłowska2008b
Leiosphaeridia crassa; Moczydłowska, fig. 2g.
- Reference Sergeev, Sharma and Shukla2008
Leiosphaeridia crassa; Sergeev et al., pl. 7, figs. 5, 6.
- Reference Yin, Yang, Peng and Kong2009
Leiosphaeridia crassa; Yin et al., figs. 3a, 3h, 3l, 4d, 4f, 4h, 5a, 5c.
- Reference Tiwari and Pant2009
Leiosphaeridia crassa; Tiwari and Pant, figs. 7d, e, 8h, 8o, p.
- Reference Stanevich, Maksimova, Kornilova, Gladkochub, Mazukabzov and Donskaya2009
Leiosphaeridia crassa; Stanevich et al., p. 32, pl. 3, figs. 3, 4.
- Reference Sergeev and Schopf2010
Leiosphaeridia crassa; Sergeev and Schopf, p. 395, fig. 15.3–15.6.
- Reference Strother, Battison, Brasier and Wellman2011
Leiosphaeridia crassa; Strother et al., fig. 1a, e.
- Reference Couëffé and Vecolii2011
Leiosphaeridia crassa; Couëffé and Vecolii, figs. 6.2, 7.1, 7.7.
- Reference Tang, Pang, Xiao, Yuan, Ou and Wan2013
Leiosphaeridia crassa; Tang et al., fig. 4b.
- Reference Lottaroli, Craig and Thusu2014
Leiosphaeridia crassa; Lottaroli et al., fig. 10.2.
- Reference Babu, Singh and Mehrotra2014
Leiosphaeridia crassa; Babu et al., fig. 3f.
- Reference Tang, Pang, Yuan, Wan and Xiao2015
Leiosphaeridia crassa; Tang et al., fig. 4d.
- Reference Nagovitsin and Kochnev2015
Leiosphaeridia crassa; Nagovitsin and Kochnev, fig. 1.55, 1.56.
- Reference Baludikay, Storme, François, Baudet and Javaux2016
Leiosphaeridia crassa; Baludikay et al., fig. 8a–c.
- Reference Porter and Riedman2016
Leiosphaeridia crassa; Porter and Riedman, p. 833, fig. 13.2, 13.6.
- Reference Sergeev, Knoll, Vorob'eva and Sergeeva2016
Leiosphaeridia crassa; Sergeev et al., fig. 4.2.
- Reference Javaux and Knoll2017
Leiosphaeridia crassa; Javaux and Knoll, p. 209, fig. 4.6.
- Reference Agić, Moczydłowska and Yin2017
Leiosphaeridia crassa; Agic et al., p. 110, fig. 8a–c.
- Reference Sergeev, Vorob'eva and Petrov2017a
Leiosphaeridia crassa; Sergeev et al., fig. 3.14.
- Reference Sergeev, Vorob'eva, Petrov and Semikhatov2017b
Leiosphaeridia crassa; Sergeev et al., pl. I, fig. 6.
- Reference Beghin, Storme, Blanpied, Gueneli, Brocks, Poulton and Javaux2017
Leiosphaeridia crassa; Beghin et al., pl. 2, figs. c, d.
- Reference Suslova, Parfenova, Saraev and Nagovitsyn2017
Leiosphaeridia crassa; Suslova et al., fig. 3.1–3.4.
- Reference Anderson, McMahon, Macdonald, Jones and Briggs2019
Leiosphaeridia crassa; Anderson et al., p. 510, fig. 8a–e.
- Reference Riedman, Porter and Calver2018
Leiosphaeridia crassa; Riedman et al., fig. 5.15.
- Reference Arrouy, Gaucher, Poiré, Xiao, Peral, Warren, Bykova and Quaglio2019
Leiosphaeridia crassa; Arrouy et al., fig. 6d, e.
- Reference Li, Pang, Chen, Zhou, Han, Yang, Wang, Yang and Yin2019
Leiosphaeridia crassa; Li et al., fig. 4f.
- Reference Arvestål and Willman2020
Leiosphaeridia crassa; Arvestål and Willman, p. 11, fig. 6j, k, m.
- Reference Knoll, Germs, Tankard and Welsink2020
Leiosphaeridia crassa; Knoll et al., p. 6, fig. 3g.
- Reference Shukla, Sharma and Sergeev2020
Leiosphaeridia crassa; Shukla et al., p. 502, fig. 6g.
- Reference Pang, Tang, Wu, Li, Chen, Wan, Yuan, Bodnar and Xiao2020
Leiosphaeridia crassa; Pang et al., fig. 2m.
For additional synonyms, also see Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) and Zang and Walter (Reference Zang and Walter1992).
Type material
Naumova (Reference Naumova1949) did not designate a holotype for Leiotriletes crassus. Subsequently, Jankauskas in Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) designated one specimen of Leiotriletes crassus published by Naumova (Reference Naumova1949) as a “holotype” (Naumova, Reference Naumova1949, pl. 1, fig. 3). In addition, he designated another specimen from a different locality and a different stratigraphic unit as a “lectotype” (Jankauskas et al., Reference Jankauskas, Mikhailova and German1989, LitNIGRI, N 16-800-2942/9, specimen 2, table 9, fig. 5). By so doing, the selection of a holotype by Jankauskas can, according to the International Code of Nomenclature for Algae, Fungi, and Plants, be taken as the designation of a lectotype (Turland et al., Reference Turland, Wiersema, Barrie, Greuter and Hawksworth2018). In addition, the specimen designated by Jankauskas as a “lectotype” should be regarded as a neotype. According to the same code, a lectotype always takes precedence over a neotype. However, the lectotype designated by Jankauskas was a specimen of Leiotriletes simplicissimus (Naumova, Reference Naumova1949), a species he synonymized with a different species of Leiosphaeridia, Leiosphaeridia minutissima. Thus, the lectotype designated by Jankauskas is not valid, and the neotype designated by Jankauskas in Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) is here considered the valid type specimen of Leiosphaeridia crassa.
Original diagnosis presented by Naumova (Reference Naumova1949) in Russian
“В очертании спора округлой или округло-овальной формы. Поверхность экзины гладкая, экзина очень толстая и плотная. Форма имеет складки смятия, щель разверзания, простая. Широко распространена в нижнем кембрии Прибалтики.”
Translation of original diagnosis presented by Naumova (Reference Naumova1949)
In outline, the spore is round or round–oval. The surface of the exine is smooth, very thick, and dense. The form has compressional folds, an opening gap, and is simple. Widespread in the lower Cambrian of the Baltic.
Emended diagnosis by Javaux and Knoll (Reference Javaux and Knoll2017) and Knoll et al. (Reference Knoll, Germs, Tankard and Welsink2020)
“A species of Leiosphaeridia with smooth, pliant walls with lanceolate folds and a modal diameter of less than 70 μm.”
Occurrence in the studied section
Fourteen specimens were recovered. They range from ~18 to ~62 μm in diameter: MP3719 and MP3720.
Illustrated specimen
CP964 (diameter ~27 μm).
Remarks
Leiotriletes crassus Naumova, Reference Naumova1949 was originally published with only a description, without a diagnosis. Although the International Code of Nomenclature for Algae, Fungi, and Plants states that either a description or a diagnosis is sufficient for the valid publication of a name (Turland et al., Reference Turland, Wiersema, Barrie, Greuter and Hawksworth2018, Art. 38.1), it is strongly recommended that both the diagnosis and description be presented when describing a new species (Hassemer et al., Reference Hassemer, Prado and Baldini2020). Later, Jankauskas in Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) reviewed some species of Leiosphaeridia and transferred Leiotriletes crassus Naumova, Reference Naumova1949 to the genus Leiosphaeridia. When Leiotriletes crassus was transferred to the genus Leiosphaerida, the species epithet was changed to crassa, so the gender of the epithet agrees with the gender of the genus name. Thus, this species became Leiosphaeridia crassa (Naumova, Reference Naumova1949) Jankauskas in Jankauskas et al., Reference Jankauskas, Mikhailova and German1989. In addition, Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) did not include in their synonym list the species Leiopsophosphaera crassa Pykhova, Reference Pykhova1973. Finally, Fensome et al. (Reference Fensome, Williams, Bars, Freeman and Hill1990), also in a work of taxonomic revision, transferred Leiopsophosphaera crassa Pykhova, Reference Pykhova1973 to Leiosphaeridia crassa (Pykhova, Reference Pykhova1973). However, Fensome et al. (Reference Fensome, Williams, Bars, Freeman and Hill1990) did not consider the study of Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) and regarded Leiotriletes crassus Naumova, Reference Naumova1949 as taxonomically uncertain (Grey, Reference Grey2005). Thus, Leiosphaeridia crassa (Pykhova, Reference Pykhova1973) is a junior homonym of Leiosphaeridia crassa (Naumova, Reference Naumova1949) Jankauskas in Jankauskas et al., Reference Jankauskas, Mikhailova and German1989. Nonetheless, Leiopsophosphaera crassa Pykhova, Reference Pykhova1973 is considered by several authors (Yin and Guan, Reference Yin and Guan1999; Grey, Reference Grey2005) as a synonym of Leiosphaeridia crassa (Naumova, Reference Naumova1949), a synonymy followed in this study. Leiosphaeridia crassa differs from Leiosphaeridia minutissima in its thicker vesicle wall, and it differs from Leiosphaeridia tenuissima and Leiosphaeridia jacutica in vesicle size (Jankauskas et al., Reference Jankauskas, Mikhailova and German1989).
Leiosphaeridia jacutica (Timofeev, Reference Timofeev1966) Mikhailova and Jankauskas in Jankauskas et al., Reference Jankauskas, Mikhailova and German1989
Figure 3.5
- Reference Timofeev1966
Kildinella jacutica Timofeev, p. 30, pl. 7, fig. 2, pl.19, fig 9, pl. 61, fig. 5, pl. 67, fig. 8, pl. 72, fig. 1.
- Reference Jankauskas, Mikhailova and German1989
Leiosphaeridia jacutica (Timofeev, Reference Timofeev1966) Mikhailova and Jankauskas in Jankauskas et al., p. 77, pl. 12, figs. 3, 7, 9.
- Reference Butterfield and Chandler1992
Leiosphaeridia jacutica; Butterfield and Chandler, fig. 5e.
- Reference Butterfield, Knoll and Swett1994
Leiosphaeridia jacutica; Butterfield et al., p. 42, fig. 16h.
- Reference Hofmann and Jackson1994
Leiosphaeridia jacutica; Hofmann and Jackson, p. 22, fig. 17.1–17.4.
- Reference Kumar and Srivastava1995
Leiosphaeridia jacutica; Kumar and Srivastava, p. 106, fig. 11k.
- Reference Sergeev2001
Leiosphaeridia jacutica; Sergeev, p. 444, fig. 8.7–8.10.
- Reference Javaux, Knoll and Walter2004
Leiosphaeridia jacutica; Javaux et al., fig. 4a–d, 4m.
- Reference Grey2005
Leiosphaeridia jacutica; Grey, p. 183, fig. 63g.
- Reference Marshall, Javaux, Knoll and Walter2005
Leiosphaeridia jacutica; Marshall et al., fig. 1c.
- Reference Prasad, Uniyal and Asher2005
Leiosphaeridia jacutica; Prasad et al., pl. 3, figs. 13, 14, pl. 4, fig. 12, pl. 9, fig. 25, pl. 10, fig. 6.
- Reference Sergeev and Seong-Joo2006
Leiosphaeridia jacutica; Sergeev and Seong-Joo, p. 14, pl. 2, fig. 6.
- Reference Javaux and Marshal2006
Leiosphaeridia jacutica; Javaux and Marshal, fig. 3.1–3.3.
- Reference Stanevich, Maksimova, Kornilova, Gladkochub, Mazukabzov and Donskaya2009
Leiosphaeridia jacutica; Stanevich et al., p. 32, pl. 3, fig. 2.
- Reference Vorob'eva, Sergeev and Knoll2009
Leiosphaeridia jacutica; Vorob'eva et al., p. 185, fig. 14.13.
- Reference Nemerov, Stanevich, Razvozzhaeva, Budyak and Kornilova2010
Leiosphaeridia jacutica; Nemerov et al., fig. 6.8, 6.9.
- Reference Prasad, Asher and Borgohai2010
Leiosphaeridia jacutica; Prasad et al., pl. 1, fig. 3.
- Reference Tang, Pang, Xiao, Yuan, Ou and Wan2013
Leiosphaeridia jacutica; Tang et al., fig. 4d.
- Reference Babu, Singh and Mehrotra2014
Leiosphaeridia jacutica; Babu et al., fig. 3l.
- Reference Chiglino, Gaucher, Sial and Ferreira2015
Leiosphaeridia jacutica; Chiglino et al., p. 643, fig. 5b.
- Reference Tang, Pang, Yuan, Wan and Xiao2015
Leiosphaeridia jacutica; Tang et al., figs. 4f, g, 5a.
- Reference Nagovitsin and Kochnev2015
Leiosphaeridia jacutica; Nagovitsin and Kochnev, fig. 4.43.
- Reference Vorob'eva, Sergeev and Petrov2015
Leiosphaeridia jacutica; Vorob'eva et al., fig. 7.6.
- Reference Baludikay, Storme, François, Baudet and Javaux2016
Leiosphaeridia jacutica; Baludikay et al., fig. 8d.
- Reference Porter and Riedman2016
Leiosphaeridia jacutica; Porter and Riedman, p. 833, fig. 13.3.
- Reference Sergeev, Knoll, Vorob'eva and Sergeeva2016
Leiosphaeridia jacutica; Sergeev et al., fig. 4.1, 4.6, 4.7.
- Reference Singh and Sharma2016
Leiosphaeridia jacutica; Singh and Sharma, p. 80, pl. 1, figs. 9, 10.
- Reference Javaux and Knoll2017
Leiosphaeridia jacutica; Javaux and Knoll, p. 209, fig. 4.4, 4.5.
- Reference Sergeev, Vorob'eva and Petrov2017a
Leiosphaeridia jacutica; Sergeev et al., fig. 3.1, 3.9–3.11.
- Reference Sergeev, Vorob'eva, Petrov and Semikhatov2017b
Leiosphaeridia jacutica; Sergeev et al., pl. I, fig. 5.
- Reference Beghin, Storme, Blanpied, Gueneli, Brocks, Poulton and Javaux2017
Leiosphaeridia jacutica; Beghin et al., pl. 2, fig. e.
- Reference Tang, Hughes, McKenzie, Myrow and Xiao2017
Leiosphaeridia crassa; Tang et al., fig. 3c.
- Reference Tang, Hughes, McKenzie, Myrow and Xiao2017
Leiosphaeridia jacutica; Tang et al., fig. 3d.
- Reference Anderson, McMahon, Macdonald, Jones and Briggs2019
Leiosphaeridia jacutica; Anderson et al., p. 12, fig. 8f–k.
- Reference Arrouy, Gaucher, Poiré, Xiao, Peral, Warren, Bykova and Quaglio2019
Leiosphaeridia jacutica; Arrouy et al., fig. 6b, c.
- Reference Li, Pang, Chen, Zhou, Han, Yang, Wang, Yang and Yin2019
Leiosphaeridia jacutica; Li et al., fig. 4h.
- Reference Arvestål and Willman2020
Leiosphaeridia jacutica; Arvestål and Willman, p. 11, fig. 6i, 6l.
- Reference Knoll, Germs, Tankard and Welsink2020
Leiosphaeridia jacutica; Knoll et al., p. 6, fig. 2g.
- Reference Shukla, Sharma and Sergeev2020
Leiosphaeridia jacutica; Shukla et al., p. 502, fig. 6l.
- Reference Pang, Tang, Wu, Li, Chen, Wan, Yuan, Bodnar and Xiao2020
Leiosphaeridia jacutica; Pang et al., fig. 2f.
- Reference Han, Chen, Li, Pang and Wang2021
Leiosphaeridia jacutica; Han et al., fig. 3a–d.
Holotype
IGD Russian Academy of Sciences no. 451/1, from upper Riphean, Lakhanda Group, Neryuen Formation, Siberia (Timofeev, Reference Timofeev1966, pl. 7, fig. 2).
Diagnosis by Javaux and Knoll (Reference Javaux and Knoll2017) and Knoll et al. (Reference Knoll, Germs, Tankard and Welsink2020)
“A species of Leiosphaeridia characterized by smooth, pliant walls with lanceolate folds and a modal diameter greater than 70 μm.”
Occurrence in studied section
Four specimens were recovered. They range from ~74 to ~98 μm in diameter: MP2990, MP3719, and MP3714.
Original description by Timofeev (Reference Timofeev1966) in Russian
“Оболочки диаметром 150–250 мк, сферические, толстые, однослойные, с поверхностью от гладкой до грубошагреневой, с резко очерченными, крупными, серповидными, иногда угловатымц складками. Цвет темно-желтый, желто-коричневый.”
Translation of original description by Timofeev (Reference Timofeev1966)
The vesicles are 150–250 microns in diameter, spherical, thick, single-layered, with a smooth to coarse shagreen surface that bears sharply defined, large, crescent-shaped, sometimes angular folds. Color dark yellow, yellow-brown.
Illustrated specimen
CP913 (diameter ~81 μm).
Remarks
Timofeev (Reference Timofeev1966) described the new species Kildinella jacutica Timofeev, Reference Timofeev1966 and designated a holotype with the description of this species, but no diagnosis was provided. Later, Mikhailova and Jankauskas in Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) proposed that Kildinella jacutica should be transferred to Leiosphaeridia jacutica (Timofeev, Reference Timofeev1966). They also designated a neotype for Leiosphaeridia jacutica. This neotype is not valid since Timofeev (Reference Timofeev1966) had designated a holotype in its publication, and there is no report of the loss of this holotype. Leiosphaeridia jacutica differs only by the larger size compared with Leiosphaeridia crassa (Jankauskas et al., Reference Jankauskas, Mikhailova and German1989). The specimen illustrated by Tang et al. (Reference Tang, Hughes, McKenzie, Myrow and Xiao2017, fig. 3) may not be Leiosphaeridia crassa but Leiosphaeridia jacutica because its diameter is around 90 μm. Leiosphaeridia jacutica differs from Bambuites erichsenii in its sphaeromorphic vesicle without processes.
Leiosphaeridia minutissima (Naumova, Reference Naumova1949) Jankauskas in Jankauskas et al., Reference Jankauskas, Mikhailova and German1989
Figure 3.1–3.3, 3.7, 3.10
- Reference Naumova1949
Leiotriletes minutissimus Naumova, p. 52, pl. 1, figs. 1, 2, pl. 2, figs. 1, 2.
- Reference Jankauskas, Mikhailova and German1989
Leiosphaeridia minutissima (Naumova, Reference Naumova1949) Jankauskas in Jankauskas et al., p. 79, pl. 9, figs. 1–4, 11.
- Reference Butterfield and Chandler1992
Leiosphaeridia minutissima; Butterfield and Chandler, fig. 3a, i.
- Reference Hofmann and Jackson1994
Leiosphaeridia minutissima; Hofmann and Jackson, p. 21, fig. 23.9–23.15.
- Reference Gaucher, Boggiani, Sprechmann, Sial and Fairchild2003
Leiosphaeridia minutissima; Gaucher and Germs, fig. 6.10–6.12.
- Reference Grey2005
Leiosphaeridia minutissima; Grey, p. 184, fig. 63d.
- Reference Blanco and Gaucher2005
Leiosphaeridia minutissima; Blanco and Gaucher, fig. 11b.
- Reference Gaucher, Poire, Peral and Chiglino2005b
Leiosphaeridia minutissima; Gaucher et al., fig. 6d.
- Reference Prasad, Uniyal and Asher2005
Leiosphaeridia minutissima; Prasad et al., pl. 9, figs. 1, 3.
- Reference Gaucher, Chiglino, Blanco, Poiré and Germs2008
Leiosphaeridia minutissima; Gaucher et al., p. 491, fig. 3a.
- Reference Moczydłowska2008a
Leiosphaeridia minutissima; Moczydłowska, p. 84, fig. 8h.
- Reference Moczydłowska2008b
Leiosphaeridia minutissima; Moczydłowska, figs. 2f, 6d.
- Reference Nemerov, Stanevich, Razvozzhaeva, Budyak and Kornilova2010
Leiosphaeridia minutissima; Nemerov et al., fig. 6.7.
- Reference Couëffé and Vecolii2011
Leiosphaeridia minutissima; Couëffé and Vecolii, fig. 7.3.
- Reference Tang, Pang, Xiao, Yuan, Ou and Wan2013
Leiosphaeridia minutissima; Tang et al., fig. 4a.
- Reference Chiglino, Gaucher, Sial and Ferreira2015
Leiosphaeridia minutissima; Chiglino et al., p. 642, fig. 5a.
- Reference Tang, Pang, Yuan, Wan and Xiao2015
Leiosphaeridia minutissima; Tang et al., fig. 4c.
- Reference Nagovitsin and Kochnev2015
Leiosphaeridia minutissima; Nagovitsin and Kochnev, fig. 4.57, 4.58.
- Reference Schopf, Sergeev and Kudryavtsev2015
Leiosphaeridia minutissima; Schopf et al., p. 724, fig. 13.10.
- Reference Baludikay, Storme, François, Baudet and Javaux2016
Leiosphaeridia minutissima; Baludikay et al., fig. 8e.
- Reference Porter and Riedman2016
Leiosphaeridia minutissima; Porter and Riedman, p. 834, fig. 13.1, 13.5.
- Reference Javaux and Knoll2017
Leiosphaeridia minutissima; Javaux and Knoll, p. 210, fig. 4.7, 4.8.
- Reference Shi, Feng, Khan and Zhu2017a
Leiosphaeridia minutissima; Shi et al., fig. 11.6, 11.7.
- Reference Beghin, Storme, Blanpied, Gueneli, Brocks, Poulton and Javaux2017
Leiosphaeridia minutissima; Beghin et al., pl. 2, figs. g, h.
- Reference Tang, Hughes, McKenzie, Myrow and Xiao2017
Leiosphaeridia minutissima; Tang et al., fig. 3a.
- Reference Suslova, Parfenova, Saraev and Nagovitsyn2017
Leiosphaeridia minutissima; Suslova et al., fig. 3.6–3.11.
- Reference Agić, Moczydłowska and Yin2017
Leiosphaeridia minutissima; Agic et al., p. 110, fig. 8g, h.
- Reference Yin, Singh, Bhargava, Zhao, Negi, Meng and Sharma2018
Leiosphaeridia minutissima; Yin et al., fig. 4h, 4j, 4l.
- Reference Javaux and Lepot2018
Leiosphaeridia minutissima; Javaux and Lepot, fig. 2e.
- Reference Lei, Shen, Algeo, Servais, Feng and Yu2019
Leiosphaeridia minutissima; Lei et al., fig. 3.13, 3.14.
- Reference Arrouy, Gaucher, Poiré, Xiao, Peral, Warren, Bykova and Quaglio2019
Leiosphaeridia minutissima; Arrouy et al., fig. 5a–g, 5j.
- Reference Li, Pang, Chen, Zhou, Han, Yang, Wang, Yang and Yin2019
Leiosphaeridia minutissima; Li et al., fig. 4e.
- Reference Shang, Liu and Moczydłowska2019
Leiosphaeridia minutissima; Shang et al., p. 24, fig. 21a.
- Reference Arvestål and Willman2020
Leiosphaeridia minutissima; Arvestål and Willman, p. 11, fig. 6c–g.
- Reference Knoll, Germs, Tankard and Welsink2020
Leiosphaeridia minutissima; Knoll et al., p. 6, fig. 2a, 2c.
- Reference Shukla, Sharma and Sergeev2020
Leiosphaeridia minutissima; Shukla et al., p. 502, fig. 6e, k, m.
- Reference Pang, Tang, Wu, Li, Chen, Wan, Yuan, Bodnar and Xiao2020
Leiosphaeridia minutissima; Pang et al., fig. 2n.
- Reference Loron, Halverson, Rainbird, Skulski, Turner and Javaux2021
Leiosphaeridia minutissima; Loron et al., fig. 6.2.
- Reference Denezine, Adôrno, Do Carmo, Guimarães, Walde, Alvarenga, Germs, Antonietto, Gianfranco and Nunes Junior2022
Leiosphaeridia minutissima; Denezine et al., fig. 11.1, 11.2.
Type material
Naumova (Reference Naumova1949) did not designate a holotype for Leiotriletes minutissimus. Afterward, Jankauskas in Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) designated one of the specimens published by Naumova (Reference Naumova1949, pl. 1, fig. 1) as the “holotype”. In addition, he designated another specimen from a different locality and a different stratigraphic unit as a “lectotype” (Jankauskas et al., Reference Jankauskas, Mikhailova and German1989, LitNIGRI, N 16-800-2942/9, table 9, fig. 1). According to the International Code of Nomenclature for Algae, Fungi, and Plants (Turland et al., Reference Turland, Wiersema, Barrie, Greuter and Hawksworth2018), the “holotype” selected by Jankauskas should be regarded as a lectotype and the “lectotype” regarded as a neotype. According to the same code, a lectotype takes precedence over a neotype. Thus, the lectotype designated by Jankauskas in Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) (Naumova, Reference Naumova1949, pl. 1, fig. 1) is the valid type specimen of Leiosphaeridia minutissima.
Diagnosis presented by Javaux and Knoll (Reference Javaux and Knoll2017)
“A species of Leiosphaeridia characterized by smooth walls with sinuous folds and a modal diameter less than 70 μm.”
Emended diagnosis presented by Knoll et al. (Reference Knoll, Germs, Tankard and Welsink2020)
“A species of Leiosphaeridia characterized by thin, smooth walls with sinuous folds and a modal diameter less than 70 μm.”
Occurrence in studied section
A total of 359 specimens were recovered. They range from ~4.4 to ~68.2 μm in diameter: MP3719, MP2977, MP2979, MP2980, MP2983, MP2985, MP2986, MP2987, MP2988, MP2992, MP2993, MP2994, MP2995, MP2998, MP2999, MP 3002, MP 3004, MP 3005, MP 3006, MP 3007, MP 3011, MP 3012, MP 3013, MP 3015, MP 3016, MP3028, MP 3030, MP3031, MP3033, MP3034, MP3035, MP3036, MP3705, MP3707, MP3708, MP3709, MP3710, MP3712, MP3713, MP3714, MP3715, MP3716, MP3719, and MP3720.
Description presented by Naumova (Reference Naumova1949) in Russian
“Очертание споры округлое. Экзина очень тонкая, прозрачная, наблюдаются многочисленные складки смятия. Поверхность экзины гладкая. Щель разверзания трехлучевая, простая, плохо различимая нз-за складок смятия.”
Translation of description presented by Naumova (Reference Naumova1949)
The outline of the vesicle is round. The exine is very thin, transparent, with numerous compressional folds. The surface of the exine is smooth. The opening slit is three-beam, simple, poorly distinguishable due to the compressional folds.
Illustrated specimens
CP918 (diameter ~32 μm), CP962 (diameters 32~ μm and ~39 μm), CP963 (diameter ~38 μm), and CP964 (diameter ~45 μm).
Remarks
The basionym of Leiosphaeridia minutissima (Naumova, Reference Naumova1949) is Leiotriletes minutissimus Naumova, Reference Naumova1949. As for Leiotriletes crassus, Naumova (Reference Naumova1949) did not present a diagnosis for this species but provided a detailed description. Subsequently, Jankauskas in Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) transferred this species to Leiosphaeridia minutissima (Naumova, Reference Naumova1949) without presenting a diagnosis. When Leiotriletes minutissimus was transferred to the genus Leiosphaerida, the epithet was changed to minutissima, so the gender of the epithet agrees with the gender of the genus name. The first formal diagnosis for Leiosphaeridia minutissima was presented by Javaux and Knoll (Reference Javaux and Knoll2017), emended later by Knoll et al. (Reference Knoll, Germs, Tankard and Welsink2020).
Leiosphaeridia tenuissima Eisenack, 1958
Figure 3.9, 3.13
- Reference Eisenack1958a
Leiosphaeridia tenuissima Eisenack, p. 391, pl. 1, figs. 2, 3.
- Reference Eisenack1958b
Leiosphaeridia tenuissima; Eisenack, pl. 2, figs. 1, 2.
- Reference Jankauskas, Mikhailova and German1989
Leiosphaeridia tenuissima; Jankauskas et al., p. 81, pl. 9, figs. 12, 13.
- Reference Butterfield, Knoll and Swett1994
Leiosphaeridia tenuissima; Butterfield et al., p. 42, fig. 16i.
- Reference Hofmann and Jackson1994
Leiosphaeridia tenuissima; Hofmann and Jackson, p. 22, fig. 15.16–15.18.
- Reference Zhang, Yin, Xiao and Knoll1998
Leiosphaeridia tenuissima; Zhang et al., p. 32, fig. 9.7.
- Reference Zhang, Yin, Xiao and Knoll1998
Leiosphaeridia spp. div.; Zhang et al., p. 32, fig. 9.8, 9.9
- Reference Turnau and Racki1999
Leiosphaeridia tenuissima; Turnau and Racki, p. 267, pl. 5, fig. 1.
- Reference Gaucher2000
Leiosphaeridia tenuissima; Gaucher, p. 68, pl. 11, fig. 5.
- Reference Gaucher and Germs2003
Leiosphaeridia tenuissima; Gaucher and Germs, fig. 6.6.
- Reference Javaux, Knoll and Walter2004
Leiosphaeridia tenuissima; Javaux et al., fig. 4j–l.
- Reference Gaucher, Chiglino and Peçoits2004
Leiosphaeridia tenuissima; Gaucher et al., fig. 4d.
- Reference Gaucher, Frimmel and Germs2005a
Leiosphaeridia tenuissima; Gaucher et al., p. 549, fig. 8g–h.
- Reference Gaucher, Poire, Peral and Chiglino2005b
Leiosphaeridia tenuissima; Gaucher et al., fig. 6a–b, 6e–h.
- Reference Blanco and Gaucher2005
Leiosphaeridia tenuissima; Blanco and Gaucher, fig. 11a.
- Reference Grey2005
Leiosphaeridia tenuissima; Grey, p. 184, fig. 63h.
- Reference Marshall, Javaux, Knoll and Walter2005
Leiosphaeridia tenuissima; Marshall et al., fig. 1d.
- Reference Prasad, Uniyal and Asher2005
Leiosphaeridia tenuissima; Prasad et al., pl. 1, fig. 3, pl. 2, fig. 10, pl. 3, fig. 15, pl. 4, fig. 17, pl. 8, figs. 16, 17.
- Reference Gaucher and Germs2006
Leiosphaeridia tenuissima; Gaucher and Germs, p. 207, figs. 7d, f, g, 8b–f.
- Reference Javaux2007
Leiosphaeridia tenuissima; Javaux, fig. 1.18, 1.19.
- Reference Gaucher, Chiglino, Blanco, Poiré and Germs2008
Leiosphaeridia tenuissima; Gaucher et al., p. 491, fig. 3b–i.
- Reference Stanevich, Maksimova, Kornilova, Gladkochub, Mazukabzov and Donskaya2009
Leiosphaeridia tenuissima; Stanevich et al., p. 32, pl. 3, fig. 5.
- Reference Prasad, Asher and Borgohai2010
Leiosphaeridia tenuissima; Prasad et al., pl. 1, fig. 1.
- Reference Buick2010
Leiosphaeridia tenuissima; Buick, fig. 1e.
- Reference Tang, Pang, Xiao, Yuan, Ou and Wan2013
Leiosphaeridia tenuissima; Tang et al., fig. 4c.
- Reference Liu, Xiao, Yin, Chen, Zhou and Li2014
Leiosphaeridia tenuissima; Liu et al., fig. 101.
- Reference Vorob'eva and Petrov2014
Leiosphaeridia tenuissima; Vorob'eva and Petrov, fig. 6b.
- Reference Schopf, Sergeev and Kudryavtsev2015
Leiosphaeridia tenuissima; Schopf et al., p. 724, fig. 13.9.
- Reference Nagovitsin and Kochnev2015
Leiosphaeridia tenuissima; Nagovitsin and Kochnev, fig. 4.59.
- Reference Chiglino, Gaucher, Sial and Ferreira2015
Leiosphaeridia tenuissima; Chiglino et al., p. 640, fig. 4a–c.
- Reference Tang, Pang, Yuan, Wan and Xiao2015
Leiosphaeridia tenuissima; Tang et al., fig. 4e.
- Reference Vorob'eva, Sergeev and Petrov2015
Leiosphaeridia tenuissima; Vorob'eva et al., fig. 7.8.
- Reference Baludikay, Storme, François, Baudet and Javaux2016
Leiosphaeridia tenuissima; Baludikay et al., fig. 8f.
- Reference Porter and Riedman2016
Leiosphaeridia tenuissima; Porter and Riedman, p. 834, fig. 13.4.
- Reference Sergeev, Knoll, Vorob'eva and Sergeeva2016
Leiosphaeridia tenuissima; Sergeev et al., fig. 4.2.
- Reference Singh and Sharma2016
Leiosphaeridia tenuissima; Singh and Sharma, p. 81, pl. 1, figs. 12, 15.
- Reference Beghin, Storme, Blanpied, Gueneli, Brocks, Poulton and Javaux2017
Leiosphaeridia tenuissima; Beghin et al., pl. 2, fig. j.
- Reference Tang, Hughes, McKenzie, Myrow and Xiao2017
Leiosphaeridia tenuissima; Tang et al., fig. 3b.
- Reference Agić, Moczydłowska and Yin2017
Leiosphaeridia tenuissima; Agic et al., p. 112, fig. 8d, f.
- Reference Suslova, Parfenova, Saraev and Nagovitsyn2017
Leiosphaeridia tenuissima; Suslova et al., fig. 3.13, 3.14.
- Reference Sergeev, Vorob'eva and Petrov2017a
Leiosphaeridia tenuissima; Sergeev et al., fig. 3.12.
- Reference Sergeev, Vorob'eva and Petrov2017a
Leiosphaeridia minutissima; Sergeev et al., fig. 3.13.
- Reference Sergeev, Vorob'eva, Petrov and Semikhatov2017b
Leiosphaeridia tenuissima; Sergeev et al., pl. 1, figs. 7, 9.
- Reference Anderson, McMahon, Macdonald, Jones and Briggs2019
Leiosphaeridia tenuissima; Anderson et al., p. 512, figs. 8l, m, 15k.
- Reference Arrouy, Gaucher, Poiré, Xiao, Peral, Warren, Bykova and Quaglio2019
Leiosphaeridia tenuissima; Arrouy et al., figs. 6a, 7a–d.
- Reference Li, Pang, Chen, Zhou, Han, Yang, Wang, Yang and Yin2019
Leiosphaeridia tenuissima; Li et al., fig. 4g.
- Reference Tang, Hu, Xie, Yuan and Wan2019
Leiosphaeridia tenuissima; Tang et al., fig. 1.2–1.5.
- Reference Wan, Tang, Pang, Wang, Bao, Meng, Zhou, Yuan, Hua and Xiao2019
Leiosphaeridia tenuissima; Wan et al., fig. 4f.
- Reference Arvestål and Willman2020
Leiosphaeridia tenuissima; Arvestål and Willman, p. 12, fig. 6a, b.
- Reference Shukla, Sharma and Sergeev2020
Leiosphaeridia tenuissima; Shukla et al., p. 502, fig. 6a–d, 6f.
- Reference Pang, Tang, Wu, Li, Chen, Wan, Yuan, Bodnar and Xiao2020
Leiosphaeridia tenuissima; Pang et al., fig. 2c.
- Reference Han, Chen, Li, Pang and Wang2021
Leiosphaeridia tenuissima; Han et al., fig. 3e.
- Reference Tang, Pang, Li, Chen, Yuan, Sharma and Xiao2021
Leiosphaeridia tenuissima; Tang et al., fig. 9a.
- Reference Loron, Halverson, Rainbird, Skulski, Turner and Javaux2021
Leiosphaeridia tenuissima; Loron et al., fig. 6.1, 6.3.
- Reference Denezine, Adôrno, Do Carmo, Guimarães, Walde, Alvarenga, Germs, Antonietto, Gianfranco and Nunes Junior2022
Leiosphaeridia tenuissima; Denezine et al., fig. 11.3.
Holotype
Preparation A3, 3 number 4, from the Dictyonema shales of the Ordovician Baltic, Nikolskaya on the Tossna, southeast Leningrad (Eisenack, Reference Eisenack1958a, pl. 1, fig. 2).
Original diagnosis presented by Eisenack (Reference Eisenack1958a) in Germany
“Wand äußerst dünn und zart, glasklar durchscheinend, ohne Wandporen; nur in flachgedrücktem Zustand in Form von fast kreisrunden Scheibchen überliefert. Pylome nicht beobachtet. Ø um rd 100 μm schwankend.”
Translation of original diagnosis presented by Eisenack (Reference Eisenack1958a)
Wall extremely thin and delicate, crystalline translucent, without wall pores; only preserved in the flattened state in the form of almost circular disks. Pyloma not observed. Size around 100 μ.
Emended diagnosis by Javaux and Knoll (Reference Javaux and Knoll2017)
“A species of Leiosphaeridia characterized by smooth walls with sinuous folds and a modal diameter (rather than maximum diameter) greater than 70 μm; the wall color is not a diagnostic criteria.”
Occurrence in the studied section
Fourteen specimens were recovered. They range from ~72 to ~126 μm in diameter: MP3002, MP3007, MP2994, MP3707, MP3709, MP3013, MP3714, MP3719, and MP3720.
Remarks
Both Leiosphaeridia tenuissima Eisenack, 1958 and Leiosphaeridia minutissima (Naumova, Reference Naumova1949,) are simple sphaeromorphs and have a thin and translucent wall less than 0.5 μm thick. However, Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989) differentiated them on the basis of vesicle size, defining specimens smaller than 70 μm in diameter as Leiosphaeridia minutissima and specimens larger than 70 μm as Leiosphaeridia tenuissima. The specimen illustrated by Sergeev et al. (Reference Sergeev, Vorob'eva and Petrov2017a, fig. 3.13) as Leiosphaeridia minutissima is better identified as Leiosphaeridia tenuissima due to its greatest diameter of about 105 μm.
Subgroup Acanthomorphitae Downie et al., Reference Downie, Evitt and Sarjeant1963
Genus Germinosphaera Mikhailova, Reference Mikhailova and Sokolov1986
Type species
Germinosphaera bispinosa Mikhailova, Reference Mikhailova and Sokolov1986.
Other species
Germinosphaera guttaformis Mikhailova in Jankauskas et al., Reference Jankauskas, Mikhailova and German1989; Germinosphaera alveolata Miao et al. (Reference Miao, Moczydłowska, Zhu and Zhu2019).
Original diagnosis presented by Mikhailova (Reference Mikhailova and Sokolov1986) in Russian
“Оболочки округлые, округло-овальные, плотные, толстые, гладкие или шагреневые, проросшие. Отростки, которые могут ветвиться, наблюдаются на одном или двух поолюсах.”
Translation of original diagnosis presented by Mikhailova (Reference Mikhailova and Sokolov1986)
The shells are round or round-oval, dense, thick, smooth or shagreen, sprouted. Processes are observed at one or two poles.
Emended diagnosis by Butterfield et al. (Reference Butterfield, Knoll and Swett1994)
“Spheroidal vesicles with 1–6 open-ended, tubular, and occasionally branched processes that communicate freely with the vesicle. Multiple processes usually restricted to a single ‘equatorial’ plane, but otherwise non-uniformly distributed on the vesicle.”
Emended diagnosis by Miao et al. (Reference Miao, Moczydłowska, Zhu and Zhu2019)
“Vesicle spheroidal, teardrop-shaped to slightly irregular outline, having psilate or low relief sculptured alveolar wall surface and bearing a single to multiple processes. Processes are simple tubular or occasionally branching, and open-ended. Processes are distributed [irregularly] on the vesicle wall, if multiple, and may be predominantly, but not exclusively, distributed in the equatorial plane of the vesicle.”
Germinosphaera bispinosa Mikhailova, Reference Mikhailova and Sokolov1986
Figure 3.4, 3.8, 3.11
- Reference Mikhailova and Sokolov1986
Germinosphaera bispinosa Mikhailova, p. 33, fig. 6.
- Reference Mikhailova and Sokolov1986
Germinosphaera unispinosa Mikhailova, p. 33, fig. 5.
- Reference Jankauskas, Mikhailova and German1989
Germinosphaera bispinosa; Jankauskas et al., p. 142, pl. 47, fig. 2.
- Reference Jankauskas, Mikhailova and German1989
Germinosphaera tadasii Weis in Jankauskas et al., p. 143, pl. 47, figs. 3–5.
- Reference Jankauskas, Mikhailova and German1989
Germinosphaera unispinosa Jankauskas et al., p. 143, pl. 47, fig. 1.
- Reference Knoll, Swett and Mark1991
Germinosphaera sp.; Knoll et al., p. 557, fig. 19.6.
- Reference Yan and Liu1993
Gemmispora rudis Yan in Yan and Liu, pl. I, figs. 6, 7.
- Reference Butterfield, Knoll and Swett1994
Germinosphaera fibrilla (Ouyang et al., Reference Ouyang, Yin and Zaiping1974); Butterfield et al., p. 38, fig. 17a–h.
- Reference Butterfield, Knoll and Swett1994
Germinosphaera bispinosa; Butterfield et al., p. 38, fig. 16d, e.
- Reference Butterfield, Knoll and Swett1994
Germinosphaera jankauskasii Butterfield in Butterfield et al., p. 38, fig. 16a–c.
- Reference Zang1995
Germinosphaera sp. cf. G. unispinosa; Zang, p. 164, fig. 26k, l.
- Reference Yin and Guan1999
Germinosphaera unispinosa; Yin and Guan, p. 128, fig. 5.2, 5.4, 5.6, 5.9.
- Reference Prasad, Uniyal and Asher2005
Germinosphaera bispinosa; Prasad et al., p. 44, pl. 11, fig. 3.
- Reference Prasad, Uniyal and Asher2005
Germinosphaera unispinosa; Prasad et al., p. 44, pl. 11, figs. 1, 2.
- Reference Yin and Yuan2007
Germinosphaera unispinosa; Yin and Yuan, fig. 2.11.
- Reference Vorob'eva, Sergeev and Knoll2009
Germinosphaera sp.; Vorob'eva et al., p. 191, fig. 13.13–13.15, 13.17.
- Reference Baludikay, Storme, François, Baudet and Javaux2016
Germinosphaera bispinosa; Baludikay et al., fig. 6a–c.
- Reference Loron and Moczydłowska2017
Germinosphaera bispinosa; Loron and Moczydłowska, p. 24, pl. 1, fig. 3.
- Reference Li, Pang, Chen, Zhou, Han, Yang, Wang, Yang and Yin2019
Germinosphaera bispinosa; Li et al., fig. 10c–g.
- Reference Loron, Rainbird, Turner, Greenman and Javaux2019
Germinosphaera bispinosa; Loron et al., fig. 8e–f.
- Reference Miao, Moczydłowska, Zhu and Zhu2019
Germinosphaera bispinosa; Miao et al., p. 187, fig. 5d, f.
- Reference Miao, Moczydłowska and Zhu2021
Germinosphaera bispinosa; Miao et al., p. 14, fig. 5d, e.
- Reference Denezine, Adôrno, Do Carmo, Guimarães, Walde, Alvarenga, Germs, Antonietto, Gianfranco and Nunes Junior2022
Germinosphaera bispinosa; Denezine et al., fig. 11.4.
Holotype
Number 882/2 from the Krasnoyarsk region, River Uderei; Upper Riphean, Dashkin Formation (Mikhailova, Reference Mikhailova and Sokolov1986, fig. 6).
Diagnosis by Butterfield in Butterfield et al. (Reference Butterfield, Knoll and Swett1994)
“A species of Germinosphaera with psilate vesicles 13–35 μm in diameter. Processes 2.5–3.5 μm wide and, when multiple, arranged equatorially on the vesicle.”
Emended diagnosis by Miao et al. (Reference Miao, Moczydłowska, Zhu and Zhu2019)
“Spheroidal to slightly elongate or irregular vesicle with one to multiple tubular processes. Vesicle wall psilate. Processes may [be] arranged irregularly or equatorially on the vesicle wall when multiple.”
Occurrence in the studied section
Twenty-three specimens were recovered: MP3036 and MP3714.
Description
Vesicles are 23.4–34.8 μm in diameter, bearing one to two processes. When two processes are present, they are inserted at two opposing ends of the vesicle (Fig. 3.4). Processes typically taper slightly toward their distal end (Fig. 3.4, lower process, 3.8) or are more or less cylindrical (Fig. 3.11). One of the processes in the specimen illustrated in Fig. 3.4 is apparently constricted at the base. However, it is uncertain whether this constriction is a taphonomic feature related to the twisting of the process. Processes are 1–3 μm in maximum diameter and 22.6–123.0 μm in preserved length.
Illustrated material
CP917.
Remarks
Mikhailova (Reference Mikhailova and Sokolov1986) established two species of Germinosphaera, Germinosphaera unispinosa and Germinosphaera bispinosa. Two additional species were published by Jankauskas et al. (Reference Jankauskas, Mikhailova and German1989), Germinosphaera guttaformis Mikhailova in Jankauskas et al., Reference Jankauskas, Mikhailova and German1989 and Germinosphaera tadasii Weiss in Jankauskas et al., Reference Jankauskas, Mikhailova and German1989. These species were distinguished by the number of processes and the psilate versus shagrinate nature of vesicle walls. However, Butterfield et al. (Reference Butterfield, Knoll and Swett1994) considered the possibility that the processes in Germinosphaera represent growth structures in vegetative stages, analogous to the modern xanthophyte Vaucheria. As such, they emended the diagnosis of Germinosphaera and the diagnosis of G. bispinosa, and they synonymized G. unispinosa with G. bispinosa. Miao et al. (Reference Miao, Moczydłowska, Zhu and Zhu2019) further emended the diagnosis of Germinosphaera and considered shagrinate vesicle walls to represent taphonomic alteration. Furthermore, they noted that the vesicle diameters of different species could overlap each other. Thus, they proposed that G. tadasii and G. jankauskasii, which are characterized by shagrinate vesicle walls, were junior synonyms of G. bispinosa. Following Miao et al. (Reference Miao, Moczydłowska, Zhu and Zhu2019), Germinosphaera currently has three species: Germinosphaera bispinosa Mikhailova, Reference Mikhailova and Sokolov1986, Germinosphaera guttaformis Mikhailova in Jankauskas et al., Reference Jankauskas, Mikhailova and German1989, and Germinosphaera alveolata Miao et al., Reference Miao, Moczydłowska, Zhu and Zhu2019.
Species diversity and abundance
The analysis of 80 samples, 53 of which contained microfossils, yielded a modest diversity of organic-walled microfossils, including seven species of four genera: Siphonophycus robustum (Schopf, Reference Schopf1968), Leiosphaeridia crassa (Naumova, Reference Naumova1949), Leiosphaeridia jacutica (Timofeev, Reference Timofeev1966), Leiosphaeridia minutissima (Naumova, Reference Naumova1949), Leiosphaeridia tenuissima Eisenack, 1958, Germinosphaera bispinosa Mikhailova, Reference Mikhailova and Sokolov1986, and Ghoshia januarensis new species. Following Butterfield et al. (Reference Butterfield, Knoll and Swett1994), the four morphospecies of Leiosphaeridia are differentiated on the basis of their vesicle diameter and wall thickness: Leiosphaeridia minutissima has thin-walled vesicles less than 70 μm in diameter, Leiosphaeridia tenuissima has thin-walled vesicles 70–200 μm in diameter, Leiosphaeridia crassa has thicker-walled vesicles less than 70 μm in diameter, and Leiosphaeridia jacutica has thicker-walled vesicles 70–800 μm in diameter (Fig. 6). Only one species of acanthomorphs is reported, Germinosphaera bispinosa, a smooth vesicle with one or two unbranched processes that are either cylindrical or slightly tapered toward the distal end.

Figure 6. Abundance and size distribution of Leiosphaeridia species from the Sete Lagoas Formation at the Barreiro section.
Filamentous microfossils are common in the Sete Lagoas Formation. Tubular filamentous microfossils recovered in this work are represented by the morphospecies Siphonophycus robustum, which is interpreted as remains of cyanobacterial sheaths. This work follows Knoll et al. (Reference Knoll, Swett and Mark1991), Butterfield et al. (Reference Butterfield, Knoll and Swett1994), and Tang et al. (Reference Tang, Pang, Xiao, Yuan, Ou and Wan2013) in distinguishing Siphonophycus species according to their filament diameter: Siphonophycus thulenema, 0.5 μm in diameter; Siphonophycus septatum, 1–2 μm; Siphonophycus robustum, 2–4 μm; Siphonophycus typicum, 4–8 μm; Siphonophycus kestron, 8–16 μm; Siphonophycus solidum, 16–32 μm; Siphonophycus punctatum, 32–64 μm; and Siphonophycus gigas, 64–128 μm. In addition to tubular filaments, branching filaments of uniserially chained cells from the Sete Lagoas Formation are identified as Ghoshia januarensis new species.
The Sete Lagoas assemblage is numerically dominated by sphaeromorphs. Nearly all fossiliferous samples contain the sphaeromorph genus Leiosphaeridia, and Leioshpaeridia minutissima is the most abundant species (Fig. 6), with 359 specimens (~93% of all Leiosphaeridia specimens) and 1–64 occurrences per horizon in 45 horizons (Fig. 7). By contrast, Leiosphaeridia crassa, Leiosphaeridia jacutica, Leiosphaeridia tenussima, and Germinosphaera bispinosa are rare, represented by 14, 4, 9, and 19 specimens, respectively. About 73% of acritarch specimens recovered from the Sete Lagoas Formation are <40 μm in diameter, highlighting the predominance of small organic-walled microfossils in this unit.

Figure 7. Stratigraphic distribution and relative abundance of organic-walled microfossils from the Sete Lagoas Formation at the Barreiro section.
The organic-walled microfossil assemblage recovered in this study is taxonomically different from those of previous micropaleontological studies of the Sete Lagoas Formation (e.g., Fairchild et al., Reference Fairchild, Schopf, Shen-Miller, Guimarães, Edwards, Lagstein, Li, Pabst and Melo Filho1996; Table 1). This difference is likely related to variations in paleoenvironment, paleoecology, taphonomy, and fossil preparation techniques. Previous micropaleontological studies of the Sete Lagoas Formation were focused exclusively on cherts, particularly silicified stromatolites and microbialites. Microfossils recovered in those studies were dominated by benthic microorganisms that constructed or dwelled in microbial mats. For example, Fairchild et al. (Reference Fairchild, Schopf, Shen-Miller, Guimarães, Edwards, Lagstein, Li, Pabst and Melo Filho1996) documented abundant filamentous and coccoidal microfossils (e.g., Siphonophycus, Myxococcoides, Gloeodiniopsis) from silicified microbialites of the Sete Lagoas Formation in the State of Goiás (their localities 20–22), more than 350 km to the west of the Barreiro section investigated in this study; only rare acritarchs identified as cf. Leioshpaeridia sp. were reported (see Fairchild et al., Reference Fairchild, Schopf, Shen-Miller, Guimarães, Edwards, Lagstein, Li, Pabst and Melo Filho1996, fig, 5h, table 2). By contrast, our investigation was focused on the lime mudstone and fine-grained limestone facies dominated by planktonic microfossils such as Leioshpaeridia minutissima. It is well known that Precambrian chert and fine-grained siliciclastic facies tend to host taxonomically distinct microfossils, with benthic microbial mat communities dominating the former facies and planktons prevailing in the latter (Butterfield and Chandler, Reference Butterfield and Chandler1992). Thus, the taxonomic difference between this and previous studies of the Sete Lagoas Formation is likely a result of paleoenvironmental and paleoecological differences.
Taphonomic differences may also have played a role in the taxonomic difference between this and previous studies. Silicification of microfossils is fundamentally a three-dimensional cast-and-mold process at the cellular level (Xiao and Tang, Reference Xiao and Tang2021), whereas organic-walled microfossils in fine-grained siliciclastic facies are preserved through two-dimensional compression of recalcitrant organic structures (Butterfield, Reference Butterfield1990), aided by clay mineral coating (e.g., Anderson et al., Reference Anderson, Schiffbauer and Xiao2011). Thus, the taxonomic difference between chert and lime mudstone and fine-grained limestone facies of the Sete Lagoas Formation is related at least partially to taphonomic variations. However, because both paleoecological and taphonomic processes are intertwined with paleoenvironmental conditions, it is impossible to disentangle the paleoecological and taphonomic factors that may have contributed to the observed taxonomic differences.
Finally, methodological differences may also have played a part in the taxonomic difference between this and previous micropaleontological studies of the Sete Lagoas Formation. Organic-walled microfossils preserved in cherts are typically observed in petrographic thin sections, whereas those in fine-grained siliciclastic rocks can be extracted for microscopic analysis. Such methodological difference can lead to biases in microfossil recovery and taxonomic identification, as shown in Ediacaran acritarchs (Xiao et al., Reference Xiao, Jiang, Ye, Ouyang, Banerjee, Singh, Muscente, Zhou and Hughes2023), which have been processed using petrographic thin sections (for those preserved three-dimensionally in cherts), hydrofluoric acid extraction (for those preserved two-dimensionally in shales), and both thin sections and acetic acid extraction (for those preserved three-dimensionally in phosphatic carbonate rocks). Therefore, the taxonomic differences between this and previous studies of the Sete Lagoas Formation likely result from a combination of paleoenvironmental, paleoecological, taphonomic, and methodological factors.
Stratigraphic distribution and biostratigraphy
Organic-walled microfossils in the Sete Lagoas Formation at the Barreiro section range from the base of the measured section to 57.5 m stratigraphic height (Fig. 7). Ghoshia januarensis has the greatest range of all species recovered in this work, occurring at nine stratigraphic levels from 0.4 m to 57.5 m. Leiosphaeridia minutissima is the longest-ranging sphaeromorph, occurring at 45 stratigraphic levels from the base of the section to 36.4 m. Leiosphaeridia tenuissima ranges from 1.3 to 36.4 m and is present in nine horizons, showing almost the same stratigraphic range as Leiosphaeridia minutissima. As a minor component of the assemblage, Leiosphaeridia jacutica was recovered from three levels in the interval of 2.8–31.5 m, Leiosphaeridia crassa from two levels in 31.5–36.4 m, Germinosphaera bispinosa from two horizons in 23.5–26.5 m, and Siphonophycus robustum from four levels in 1.9–28.0 m.
Except for Leiosphaeridia crassa and Germinosphaera bispinosa, all recovered species have their first appearance within 2 m above the base of the studied section, where there is a predominance of lime mudstone. Leiosphaeridia crassa and Germinosphaera bispinosa first emerge in the middle part of the section below the intraclastic breccia beds. The disappearance of organic-walled microfossils in the Sete Lagoas Formation is gradual, although three species (Leiosphaeridia crassa, Leiosphaeridia minutissima, and Leiosphaeridia tenuissima) disappear at approximately 37 m. No organic-walled microfossils other than Ghoshia januarensis were recovered above 37 m, right before a great abundance of intraclastic breccias, which were interpreted as seismic deposits by Okubo et al. (Reference Okubo, Warren, Luvizotto, Varejão, Quaglio, Uhlein and Assine2020).
With the exception of Ghoshia januarensis, organic-walled microfossils from the Sete Lagoas Formation described in this paper have very long stratigraphic ranges when global data are considered. For example, the four Leiosphaeridia species recovered in this work range from the Mesoproterozoic to the Cambrian (Grey, Reference Grey2005). Both Germinosphaera bispinosa and Siphonopycus robustum are known from the late Paleoproterozoic to the Paleozoic (Butterfield et al., Reference Butterfield, Knoll and Swett1994; Sergeev et al., Reference Sergeev, Sharma and Shukla2012; Miao et al., Reference Miao, Moczydłowska, Zhu and Zhu2019).
Because leiospheric sphaeromorphs have rather long stratigraphic ranges globally, they have limited utility for global biostratigraphic correlation, which casts doubt on the biostratigraphic significance of leiosphere-based biozones. Nonetheless, Grey (Reference Grey2005) established the Ediacaran Leiosphere Palynoflora, and Gaucher and Sprechmann (Reference Gaucher and Sprechmann2009) proposed the Early Ediacaran Leiosphere Palynoflora. Both were regarded as early Ediacaran (ca. 635–580 Ma) acritarch biozones. More recent studies from South China, however, recovered abundant and diverse acanthomorphs from early Ediacaran strata (Zhou et al., Reference Zhou, Xie, McFadden, Xiao and Yuan2007; Liu and Moczydłowska, Reference Liu and Moczydłowska2019; Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021), indicating that the leiosphere-based biozones of Grey (Reference Grey2005) and Gaucher and Sprechmann (Reference Gaucher and Sprechmann2009) are controlled by local environmental, regional biogeographical, or taphonomic factors.
It is perceived that the terminal Ediacaran (ca. 550–539 Ma) is characterized by a leiosphere assemblage (Knoll and Walter, Reference Knoll and Walter1992; Gaucher and Sprechmann, Reference Gaucher and Sprechmann2009) (Fig. 17). Gaucher and Sprechmann (Reference Gaucher and Sprechmann2009) presented the Late Ediacaran Leiosphere Palynoflora, which is a low-diversity assemblage characterized by small sphaeromorphs (<150 μm) such as Leiosphaeridia minutissima and Leiosphaeridia tenuissima, among others (Fig. 17). In addition, there are occurrences of Chuaria circularis, as well as Bavlinela faveolata Shepeleva, Reference Shepeleva1962, Soldadophycus bossii Gaucher et al., Reference Gaucher, Sprechmann and Schipilov1996, and small acanthomorphs, such as Asteridium spp. The Late Ediacaran Leiosphere Palynoflora, sensu Gaucher and Sprechmann (Reference Gaucher and Sprechmann2009), has been documented in the Nama Group in Namibia (Germs et al., Reference Germs, Knoll and Vidal1986), the Holgat Formation of the Port Nolloth Group in Namibia (Gaucher et al., Reference Gaucher, Frimmel and Germs2005a), the Mulden Group in Namibia (Gaucher and Germs, Reference Gaucher and Germs2007), the Tent Hill Formation in Australia (Damassa and Knoll, Reference Damassa and Knoll1986), Cijara Formation in Spain (Palacios, Reference Palacios1989), the Cango Caves and Gamtoos groups in South Africa (Gaucher and Germs, Reference Gaucher and Germs2006), the Dengying Formation in South China (Yin and Yuan, Reference Yin and Yuan2007), the Arroyo del Soldado Group in Uruguay (Gaucher, Reference Gaucher2000; Gaucher et al., Reference Gaucher, Boggiani, Sprechmann, Sial and Fairchild2003), the Sierras Bayas Group in Argentina (Cingolani et al., Reference Cingolani, Rauscher and Bonhomme1991; Gaucher et al., Reference Gaucher, Poire, Peral and Chiglino2005b), the La Providencia Group in Argentina (Arrouy et al., Reference Arrouy, Gaucher, Poiré, Xiao, Peral, Warren, Bykova and Quaglio2019), and the Corumbá Group in Brazil (Zaine, Reference Zaine1991; Gaucher et al., Reference Gaucher, Boggiani, Sprechmann, Sial and Fairchild2003; Tobias, Reference Tobias2014). In Namibia, Argentina, Uruguay, and Brazil (Germs et al., Reference Germs, Knoll and Vidal1986; Gaucher et al., Reference Gaucher, Boggiani, Sprechmann, Sial and Fairchild2003, Reference Gaucher, Poire, Peral and Chiglino2005b; Tobias, Reference Tobias2014), the Late Ediacaran Leiosphere Palynoflora occurs in association with biomineralized tubular fossils such as Cloudina lucianoi (Beurlen and Sommer, Reference Beurlen and Sommer1957), Cloudina riemkeae Germs, Reference Germs1972, and Corumbella werneri Hahn et al., Reference Hahn, Hahn, Leonardos, Pflug and Walde1982, which are potential index fossils for the terminal Ediacaran.
Given the predominance of a depauperate leiosphere assemblage in the Sete Lagoas Formation (this study) and the previous report of Cloudina and Corumbella from this unit (Warren et al., Reference Warren, Quaglio, Riccomini, Simões, Poiré, Strikis, Anelli and Strikis2014), it is tempting to consider that the organic-walled microfossil assemblage reported in this study is correlated with the Late Ediacaran Leiosphere Palynoflora. There are, however, two caveats. First, as discussed by Xiao and Narbonne (Reference Xiao, Narbonne, Gradstein, Ogg, Schmitz and Ogg2020), several recent studies have shown acanthomorphs as a group may extend from the lowermost Ediacaran to the terminal Ediacaran stage in Mongolia (Anderson et al., Reference Anderson, Macdonald, Jones, McMahon and Briggs2017, Reference Anderson, McMahon, Macdonald, Jones and Briggs2019) and in Siberia (Golubkova et al., Reference Golubkova, Zaitseva, Kuznetsov, Dovzhikova and Maslov2015; but see Vorob'eva et al., Reference Vorob'eva, Sergeev and Knoll2009), to rocks considered younger than the Shuram Excursion (Ouyang et al., Reference Ouyang, Guan, Zhou and Xiao2017) or even to the early Cambrian (Grazhdankin et al., Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020). Second, a systematic description of the purported Cloudina and Corumbella fossils from the Sete Lagoas Formation (Warren et al., Reference Warren, Quaglio, Riccomini, Simões, Poiré, Strikis, Anelli and Strikis2014; Perrella Júnior et al., Reference Perrella Júnior, Uhlein, Uhlein, Sial, Pedrosa-Soares and Lima2017) is needed to assess their species-level identification and to support their biostratigraphic significance. Nonetheless, considering the maximum age constraint of ~557 Ma provided by detrital zircons from the upper Sete Lagoas Formation (Paula-Santos et al., Reference Paula-Santos, Babinski, Kuchenbecker, Caetano-Filho, Trindade and Pedrosa-Soares2015), the organic-walled microfossil assemblage reported in this paper is consistent with a terminal Ediacaran age interpretation.
Conclusions
A modest diversity of organic-walled microfossils is reported from the Sete Lagoas Formation of Bambuí Group at the Barreiro section in the Januária area of the São Francisco basin, central Brazil. Seven species are described: Siphonophycus robustum (Schopf, Reference Schopf1968), Ghoshia januarensis new species, Leiosphaeridia crassa (Naumova, Reference Naumova1949), Leiosphaeridia jacutica (Timofeev, Reference Timofeev1966), Leiosphaeridia minutissima (Naumova, Reference Naumova1949), Leiosphaeridia tenuissima Eisenack, 1958, and Germinosphaera bispinosa Mikhailova, Reference Mikhailova and Sokolov1986. The first two species are considered cyanobacteria, the four Leiosphaeridia species are considered possible protists, and the phylogenetic affinity of Germinosphaera bispinosa is uncertain. All species occur in the lower part of the studied section, but only Ghoshia januarensis extends to the upper portion of the studied section. The assemblage is numerically dominated by Leiosphaeridia, with Leiosphaeridia minutissima being the most abundant species. The predominance of Leiosphaeridia indicates that the Sete Lagoas organic-walled microfossil assemblage may be correlated with the Late Ediacaran Leiosphere Palynoflora, consistent with a terminal Ediacaran age interpretation inferred from detrital zircon data, Cloudina, and Corumbella from the Sete Lagoas Formation. However, we emphasize that further investigation is needed to test this age interpretation.
Acknowledgments
We thank all institutions that participated in the development of this research: the National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), Geological Survey of Brazil (CPRM), Brazilian Petroleum Corporation (PETROBRAS), National Agency of Petroleum, Gas, and Biofuels (ANP), and the University of Brasília (UnB). We thank FINATEC for assistance in administrative affairs supporting scientific projects in Brasília. We thank A.H. Giles for grammar review and the undergraduate geology students L.R.O. Gonçalve and A.S. Reis for curating samples at UnB. This study was financed in part by the Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES)—Finance Code 001. S.X. acknowledges the support from the National Science Foundation (EAR-2021207). C. Z. acknowledges the support from São Paulo Research Foundation (2021/12304-0).
Declaration of competing interests
The authors declare none.











